Fabio Giancane1, Angelo Cianciulli1, Silvia De Chiara2, Alessandra Iannelli2,
Marika Finizio4, Rosetta Frammartino1, Andrea Lombardi1,
Domenico Ciro Cristiano1, Francesco Gravante3, Francesco Petrosino1*
1.Nurse, AOU ‘San Giovanni di Dio e Ruggi d’Aragona’, Salerno
2.Pharmacist, AOU ‘San Giovanni di Dio e Ruggi d’Aragona’, Salerno
3.Nurse, Local Health Unit of Aversa, Caserta
4.Nursing graduate, University of Salerno
*Corresponding author: Francesco Petrosino, AOU ‘San Giovanni di Dio e Ruggi d’Aragona’, Salerno. Email: f.petrosino75@gmail.com
Cite this article
ABSTRACT
Introduction: In Italy, approximately 80.5% of the population has completed the primary anti-COVID vaccination cycle with approximately 141 million doses administered. With the introduction of new measures to counter the spread of COVID-19, including compulsory vaccination for certain categories of people, the population expressed fears about the safety and adverse effects of SARS-CoV-2 vaccines. Several factors, such as gender and age, could have influenced the outcomes associated with the vaccine. Our single-centre work seeks to provide such evidence with respect to Pfizer/BioNTech’s BNT162b2 (Comirnaty) and AstraZeneca’s AZD1222 (Vaxzevria) vaccines.
Materials and Methods: Single-centre descriptive study carried out on a sample of subjects who underwent anti-COVID vaccination at the ‘San Giovanni di Dio e Ruggi d’Aragona’ AOU vaccination centre in Salerno. Patients who reported a suspected adverse reaction after receiving a dose of vaccine were included in the study. The regional vaccine platform SORESA and the VigiFarmaco portal were used to collect the data.
Results: During the period covered by the study, 126,928 doses of SARS-CoV-2 vaccine were administered. The Pfizer-BioNTech vaccine group comprised 124,138 administrations. The AstraZeneca vaccine group consisted of 2,790 administrations. 287 post-vaccination adverse reaction reports entered in the National Pharmacovigilance Network were considered. In most of the reactions reported, for both vaccines considered, the symptomatology was attributable to local reactions at the injection site. At the systemic level, however, we noted the prevalence of non-specific events such as fever, headache and diffuse arthromyalgia.
Conclusions: Based on our results and comparison with the literature, the data collected on the vaccines considered in the study suggest a favourable safety profile for their large-scale use. The rate of minor adverse events turned out to be low, with similarly reassuring data compared to serious adverse events, such as not to justify hesitation towards vaccination for COVID-19 disease control.
Keywords: SARS-CoV-2; Surveillance system; COVID-19 vaccination; mRNA; Viral vector; Adverse events following immunisation
INTRODUCTION
Pathogenic viral outbreaks and complex interactions with humans and animals have, over the centuries, caused the transmission of viruses between different species (jumping), posing a great threat to human health and safety[1-3]. Globalisation has increasingly favoured pathogenic transmission between continents, causing different pandemics, in particular viral pandemics[4]. A new public health crisis that threatened the world in 2019 was the spread of the new coronavirus (2019-nCoV) or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) declared a pandemic by the World Health Organisation (WHO) in March 2020[5]. The rapid spread of the COVID-19 disease has focused researchers’ attention on the repurposing of existing approved drugs that inhibit viral entry, endocytosis, genome assembly, transmission and replication[6]. Most of the available information has been obtained through studies on other members of this family (SARS and MERS)[7]. Many researchers are currently working on the development of various types of specific drugs to treat this disease worldwide[8, 9]. Therefore, the current treatment given to COVID-19 patients is only based on their symptoms[10, 11]. Exposure to a pathogen such as SARS-CoV-2 generates an antibody response that changes over time and in different individuals (antibody kinetics)[12, 13]. It is believed that the limited pre-existing natural immunity to this virus was responsible for the explosive increase in cases[14, 15]. A previous infection, on the other hand, could play a key role in ensuring protection against new infections, and the literature can provide evidence of such protective correlations through longitudinal cohort studies[16-19]. In the absence of specific drugs, only global vaccination has made it possible to contain the spread of the virus, reducing the number of serious clinical cases and hospitalisations[20-22]. In December 2020, the first vaccines against COVID-19 developed with different technologies received Emergency Use Authorisation (EUA) from the US Food and Drug Administration (FDA). Subsequently, globally, they were licensed in 117 countries in North and South America, the United Kingdom, Europe, Africa, Asia and Oceania[23, 24].
In Italy, mRNA vaccines and the adenovirus vaccine AZD1222 have been widely administered. Time saving during the development of COVID-19 vaccines was achieved through unprecedented levels of public financial support, increased tolerance for risky investments in technology and process, and studies on mRNA transport methodology[25-27]. The Italian Medicines Agency defines pharmacovigilance and vaccine vigilance as “a complex set of activities aimed at continuously assessing all information relating to the safety of medicinal products and ensuring that the benefit/risk (B/R) ratio remains favourable over time.” Our country has a pharmacovigilance system that, for many years now, has devoted special attention and a special organisational structure precisely to monitoring what happens after a vaccine is administered. It is an open, dynamic system to which everyone (health professionals, patients, parents, citizens) can send their reports contributing to the monitoring of the safe use of vaccines and medicines in general. Furthermore, the system has full transparency and offers access to aggregated data, which can be queried on the AIFA website. The National Pharmacovigilance Network (NFP) suddenly came into the spotlight when several new vaccines received emergency authorisation and were launched on a large scale at the end of 2020. Vaccines have undergone rigorous clinical testing and evaluation by the authorities, but with the use of new technologies [28] and rapid, large-scale administration of vaccines, the importance of a well-functioning international system of post-marketing safety surveillance has been emphasised[29, 30]. The surveillance of vaccine safety and the collection of reports on suspected adverse events after immunisation (AEFI) [31] is the responsibility of national vaccine regulatory systems, including national regulatory authorities (NRAs) and national immunisation programmes (NIPs). Passive surveillance, defined as the collection and analysis of unsolicited reports of suspected adverse events in the form of individual case safety reports (ICSRs) that are sent to a central database or a health authority, is the basis of safety surveillance for immunisation programmes, in order to identify rare events, evaluate clusters of reports and detect safety signals for further and subsequent studies [32, 33]. Although the identification and quantification of adverse events related to anti-COVID vaccination is not always easy to understand, especially in such a broad context as a pandemic, the analysis of the available data can be an important moment for risk estimation and subsequent safety assessments[34-36].
This paper describes reports of reactions that were observed after administration of the COVID vaccine. Investigating the meaning and causes of these reactions is the task of pharmacovigilance. Investigating every event that appears after a vaccination serves to gather as much information as possible and increase the possibility of identifying truly suspicious events whose nature is important to understand, or which have never been observed before, with the aim of ascertaining whether there is a causal link with the vaccination. In this way, regulatory authorities such as AIFA can verify the safety of vaccines in the real world, confirming what has been observed in pre-authorisation studies and possibly identifying new potential adverse reactions, especially if they are rare (1 in 10,000) and very rare (less than 1 in 10,000). A large number of reports, therefore, does not imply that the vaccine is more dangerous, but is an indication of the high capacity of the pharmacovigilance system to monitor safety.
The anti-Sars-CoV-2 vaccination campaign, which started on 27 December 2020, saw the participation of the ‘San Giovanni di Dio e Ruggi d’Aragona’ AOU of Salerno in ‘Vaccine Day’, the symbolic start date of the vaccination campaign in Italy and across Europe. In what was analysed by this work, in order to make this event – historic for all healthcare worldwide – possible, an organisational and coordination process was implemented that ensured high daily vaccination numbers and minimal risks for users, in full compliance with the quality standards of Public Health.
Several factors, such as gender and age, may have influenced the clinical outcomes associated with the vaccine[37]. To date, in Italy, about 80.5% (48 million subjects) of the population have completed the COVID-19 primary vaccination cycle with about 141 million doses administered. This followed the introduction of new measures to combat the spread of COVID-19, including the compulsory vaccination of certain categories of persons[38].
MATERIAL AND METHODS
Study design
In this paper, we will provide a surveillance report on vaccines administered at the ‘San Giovanni di Dio e Ruggi d’Aragona’ University Hospital (AOU ‘Ruggi’) in Salerno with respect to specific targets:
1.to conduct a descriptive observational study of subjects undergoing vaccination with Pfizer/BioNTech’s BNT162b2 (Comirnaty) and AstraZeneca’s AZD1222 (Vaxzevria) between 27 December 2020 and 30 November 2021
2.to conduct a descriptive analysis of all reports of suspected adverse drug reactions attributed to COVID-19 vaccination (Adverse Events and Severe Adverse Events Following Immunisation, AEFI and sAEFI), collected through the AIFA form and/or the VigiFarmaco system of the Italian Drug Agency (severity, concomitant use of drugs, outcome)
3.to assess the role and statistical association between reported reactions and previously identified variables (age, gender, dose).
Participants
The descriptive observational study was carried out on a sample of subjects who received the vaccine at the ‘San Giovanni di Dio e Ruggi d’Aragona’ AOU vaccination centre in Salerno in the period between 27 December 2020 and 30 November 2021. Patients who reported a suspected adverse reaction after receiving a dose of vaccine were included in the study.
Sample Size
The sample size was evaluated using the GPower software version 3.1.9.7[39]. Power analysis was conducted for a two-tailed t-test, with an effect size = 0.50, a probability of type I error = 0.05, a test power = 0.95 and a sample size ratio of 1:1. The sample size for group 1 is 105 and for group 2 it is 105 for a total of 210 items. For the goodness-of-fit χ2 test, with an effect size = 0.3, a probability of the type 1 error = 0.05, a power of the test = 0.95 and GdL = 1, the sample size for the group is 143 (172 with 2 degrees of tolerance).
Data collection
For the vaccination population, data were obtained from the SINFONIA platform (Sistema Informativo Sanità Campania) and the VigiFarmaco portal for adverse event reporting. They were collected anonymously, formatted, narrowed down to the vaccines of interest for this study and entered into a database using Microsoft Corporation Excel software.
Data analysis
The collected data were processed with SPSS ® (Statistical Package for Social Science – Chicago, IL, USA) statistical software for Windows, version 26.0. A descriptive analysis of the general characteristics of the study population was performed, using absolute frequencies and percentages. Data were stratified by age group, gender and period of administration. For continuous variables, results were expressed as mean ± standard deviation (SD), and as median and Interquartile range (RIQ) for numeric variables. Paired and unpaired data were analysed using Student’s t-test. The Kolmogorov-Smirnov test, which is more appropriate when the sample size is >50, was used to check normality. The Q-Q diagram and the values of skewness and kurtosis were also evaluated. The categorical variables were summarised using frequencies and percentages, and we used the chi-square (χ2) test to compare the categorical variables between the groups.
The Phi coefficient was used to measure the strength of association between the dichotomous variables, while Pearson’s linear correlation coefficient was used to assess the degree of relationship between the variables age and severity.
All tests with p-value (p) < 0.05 were considered statistically significant.
Ethical consideration
Due to the nature of this study, no formal approval to the relevant Ethics Committee was required. The study was conducted in accordance with the principles of the Declaration of Helsinki. The data were extracted from databases for which the processing information had been signed in advance and analysed for the time strictly necessary to achieve the purposes for which they were collected, in compliance with the Regulation (EU) 2016/679 (GDPR) on privacy and guarantee of anonymity. Authorisation for their use was provided by the Corporate Privacy Officer and the legal representative of the organisation.
RESULTS
During the period covered by the study, 126,928 doses of SARS-CoV-2 vaccine were administered. Those who had already received the first dose went to the centre for the administration of the second dose of vaccine. Some of them also received the third dose.
In Table 1, the general trend of the Vaccination Centre is shown, while in Table 2, the trend per macro area (Vaccination Type and Dose) is shown.

Table 1. General performance of the Vaccination Centre

Table 2. Performance by macro area of the Vaccination Centre, with order of priority of categories
A proportion of the vaccinated subjects (404) switched from AstraZeneca to Pfizer-BioNTech due to changes in the Italian Regulatory Authority’s declarations [40] or because they had suffered increased D-dimer levels [41]after the first AZD dose.
The Pfizer-BioNTech vaccine group (BNT) comprised 124,138 administrations (47.8% men and 52.2% women) with a mean age of 49.11±20.94 years (range: 12-101) and a median age of 51 years (RIQ: 31-64).
The distribution is superimposable between first dose (50.6%) and second dose (47.2%). Only 2.2% of the subjects received the third dose.
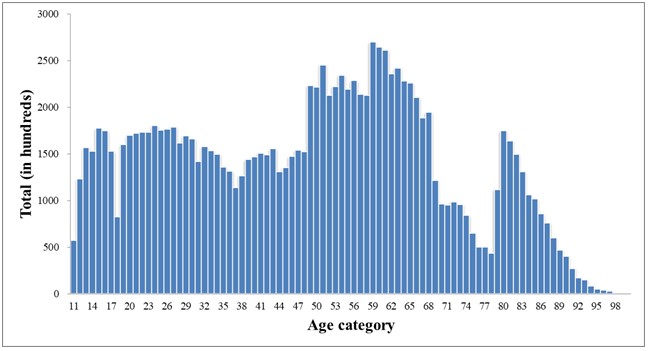
Figure 1. Age distribution BNT Group
The AstraZeneca vaccine group (AZD) consisted of 2,790 administrations (57.7% men and 42.3% women), with a mean age of 47.46±11.77 (range: 18-76 years) and a median age of 49 years (RIQ: 38-57). 58.7% of the sample received the first dose and 41.3% the second dose.

Figure 2. Age distribution AZD Group
The enrolled population was stratified by age decades. The Pfizer-BioNTech group consisted predominantly (18.9%) of individuals aged between 60 and 69 years (Figure 3), whereas the AstraZeneca group comprised more people (23.1%) aged between 40 and 49 years (Figure 4). In particular, several subjects were unable to receive AstraZeneca mainly due to thrombotic risk (e.g. high D-dimer value, coagulation impairment, etc.) and age limitation (initially subjects over 18 years of age were eligible and later over 60 years of age), according to the recommendations in force in Italy. Our study therefore examined the AEFIs and sAEFIs attributed to the SARS-CoV-2 vaccination and recorded by the nursing staff or pharmacists responsible for vaccine preparation and pharmacovigilance at the AOU ‘San Giovanni di Dio e Ruggi d’Aragona’ Vaccine Centre.
At the time of its closure on 30 November 2021, we verified that 287 post-vaccination adverse reaction reports had been entered into the National Pharmacovigilance Network. The data show that the percentage of reported sAEFIs are significantly lower than the risks related to COVID-19 (data from the COVID-19 Integrated Surveillance in Italy).
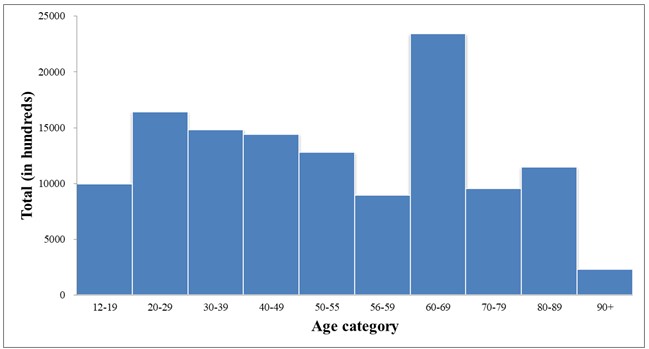
Figure 3. Distribution by age group BNT Group

Figure 4. Age distribution AZD Group
Most of the reported adverse events were classified as non-serious (90.9%) and to a lesser extent as serious (9.1%); in most cases, the outcome was complete resolution or improvement of symptoms. The distribution of reports per type of vaccine follows the distribution of administrations (92% for Pfizer-BioNTech and 8% for AstraZeneca).
The average age of persons reporting a suspected adverse event was 62±22.21 years (range: 15-99). As already observed in the National Surveillance Reports on anti-COVID-19 vaccines, also in what was analysed by this work, the reporting rates for the 2nd dose are lower than for the 1st dose and significantly lower for the 3rd dose. Although these are not absolute incidence rates, the data indicate an overall absence of significant sAEFI events such as to be an alert for regulators to serious safety issues with administered vaccines. On the other hand, in contrast to the overlapping exposure between the genders (52% of doses administered in females and 48% in males), one can note the asymmetric distribution of reports with respect to gender, with 71.1% of reports concerning women and 27.9% concerning men, regardless of the vaccine and the dose administered.
In our statistical analysis, in order to determine whether there were age differences between those who reported a post-vaccination adverse reaction, we performed an independent samples t-test.
To test for normality, we used the Kolmogorov-Smirnov test with Lilliefors correction, which was non-significant for both men (p = 0.071) and women (p = 0.058). Visual inspection of the Q-Q plot shows that age is normally distributed, with a skewness of -0.445 (ES = 0.271, | z | = 1.64) and kurtosis of -0.634 (ES = 0.535) for men and a skewness of 0.006 (ES = 0.171, | z | = 0.035) and kurtosis -0.902 (ES = 0.341) for women[42-43]. Having ascertained the normality of the sample distribution, we evaluated the hypothesis of equality of variance by means of Levene’s test. This turns out to be statistically non-significant (F = 0.255, p = 0.614) and it is therefore possible to use the assumption of homogeneous variance of the age of males and females.
The results suggest that the difference in mean age between the two groups is not significantly different (t(279) = 1.769, p = 0.078). We then looked at whether the 287 subjects in our sample were as likely as the Italian population to have non-serious (81.8%) or serious (18.1%) reactions[44].
We conducted a χ2 test for goodness of fit against the theoretical distribution model. In this case, the results obtained suggest that the two categories do not distribute themselves according to the expected probability (χ2(1) = 15.88, p < 0.001). In particular, in our reference sample, non-serious reactions are more frequent (90.9%) than serious reactions (9.1%). We then conducted a χ2 test to test whether men and women were equally likely to have non-serious or serious reactions. The test results suggest that men and women were equally distributed within the two categories of the severity status variable (χ2(1) = 2.83, Phi = -0.100, p = 0.093). In other words, there is no evidence of linear dependence between gender and the occurrence of a serious adverse reaction, with a small linkage effect between the two variables, as suggested by the value close to 0 for the Phi coefficient. We also conducted a χ2 test to test whether there was a relationship between the number of doses received and the occurrence of a severe reaction. These results also suggest that the groups are equally distributed within the two categories of the severity status variable (χ2(2)= 0.418, p = 0.811, V = 0.038). In other words, dose and severity of the reaction are independent in distribution. Finally, Pearson’s linear correlation coefficient was calculated to investigate the correlation between the variables age and severity considering the reported adverse reaction (r = -0.279, p = 0.01).
The results suggest that as age decreases, there is a weak correlation with the presentation of a severe reaction. In this analysis, the source variable (severity) was coded into a nominal dichotomous qualitative variable with value 0 (no severe reaction) and 1 (severe reaction).
Below are some of the reactions detected and their incidence in relation to the total number of detections (Figure 5).
Figure 6 shows some detected reactions and their incidence for the Pfizer-BioNTech vaccine and Figure 7 for AstraZeneca.

Figure 5. All types of adverse events observed on our sample.

Figure 6. All types of adverse events observed by Comirnaty vaccine.

Figure 7. All types of adverse events observed by Vaxzevria vaccine.
Finally, Figure 8 shows the reports of sAEFI aggregated by symptomatology. It can be noted that,

Figure 8. All types of severe adverse events observed.
Figure 8 shows the reports of severe AEFIs aggregated by symptomatology. It can be seen that, in addition to overlapping with the AEFIs in terms of typology, they are characterised by events attributable to general pathologies. For all reported sAEFIs, the outcome was improvement of symptoms.
DISCUSSIONS
Our study examined AEFIs and sAEFIs spontaneously reported at the AOU ‘San Giovanni di Dio e Ruggi d’Aragona’ in Salerno through the AIFA pharmacovigilance system and attributed to anti-COVID vaccination. Our data, although related to a small sample, demonstrate the few reports of serious reactions and the low risk of outcomes when compared to historical pandemic data and in line with national data. In contrast to an overlapping exposure between the sexes with respect to total administrations, AEFIs were predominantly reported in the female sex (71%). The percentage of sAEFI is almost overlapping between the sexes, with a prevalence for Pfizer-BioNTech’s Comirnaty vaccine (78%) at the first dose (77%). In most of the AEFIs reported, for both vaccines considered, the symptomatology was attributable to local reactions at the injection site (e.g. pain, swelling, redness). At the systemic level, however, we noted the prevalence of non-specific events such as fever, headache and diffuse arthromyalgia. The same applies to reactions reported as serious; the latter, identified as such due to the prolonged observation period at the vaccination centre, in rare cases led to the hospitalisation of those involved. All these reports resulted in an improvement in symptoms. In line with the literature, our study showed that the onset of AEFI can be influenced by gender. This could be related to the opposite role of sex hormones [42] as well as pharmacokinetic parameters that may differ between males and females [43].
Disease control efforts by health authorities should seriously consider the relationship between the risks involved in immunising the population versus the benefits against the disease[45]. While there is no general acceptable risk threshold, the number of deaths worldwide from COVID-19, the risk of collapse of health systems, shutdowns and damage to economies, should lead epidemiologists, health organisations and governments to set this threshold as soon as possible.
CONCLUSIONS
Based on our results and the comparison with the literature[46, 47], both vaccines showed a favourable safety profile, with reassuring data that does not justify hesitation towards vaccination for COVID-19 disease control[48-50]. We therefore highlighted the few differences in the incidence and type of AEFI and sAEFI associated with Pfizer/BioNTech’s Comirnaty (BNT162b2) and AstraZeneca’s Vaxzevria (AZD1222). For these reasons, the guidelines issued by many countries, such as Italy, whose main objective is to increase the number of vaccinated persons with a ‘fourth dose’ to protect the over 60s and the frailest of the population. It is therefore necessary to disseminate surveillance and public health data to counter vaccination hesitancy in the general population and the reluctance of “no vax” subjects towards vaccinations, also in view of possible future pandemic events.
LIMITS
The study has some limitations. Pharmacovigilance information is based on a voluntary and passive reporting system that may not capture every single event related to AEFI and sAEFI. Direct verification is not always possible to determine whether every reported adverse reaction is actually related to the vaccine. In particular, the lack of reporting could lead to an underestimation of all the adverse events that actually occurred.
Another limitation of the study is that, to the exclusion of age and gender, other individual characteristics were not taken into account, such as underlying or previous diseases (myocarditis, autoimmune or immune-mediated diseases, oncological pathologies) or chronically taken medications, which might instead predispose vaccinated subjects to be susceptible to AEFIs and sAEFIs.
ACKNOWLEDGEMENTS
We thank Prof Francesco De Caro (UOC Risk Assessment Management and Reporting) and Dr Maria Grazia Lombardi (UOC Pharmacy), AOU ‘San Giovanni di Dio e Ruggi d’Aragona’, Salerno.
FUNDING
This research did not receive any external funding or support.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest associated with the study.
AUTHORS’ CONTRIBUTION
FG and AC were responsible for the conception and design of the study; SDC and AI performed the data collection; FG and FP performed the data analysis; MF and RF were responsible for drafting the manuscript; AL and DCC made critical revisions to the article.
REFERENCES
- Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents. 2020;56(2):106054.
- Reperant LA, Osterhaus ADME. AIDS, Avian flu, SARS, MERS, Ebola, Zika… what next?. Vaccine. 2017;35(35 Pt A):4470-4474.
- Reperant LA, Cornaglia G, Osterhaus AD. The importance of understanding the human-animal interface: from early hominins to global citizens. Curr Top Microbiol Immunol. 2013; 365:49-81.
- Liu J, Dai S, Wang M, Hu Z, Wang H, Deng F. Virus like particle-based vaccines against emerging infectious disease viruses. Virol Sin. 2016;31(4):279-287.
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern [published correction appears in Lancet. 2020 Jan 29;:]. Lancet. 2020;395(10223):470-473.
- Muralidar S, Ambi SV, Sekaran S, Krishnan UM. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie. 2020;179:85-100.
- Chan-Yeung M, Xu RH. SARS: epidemiology. Respirology. 2003;8 Suppl(Suppl 1):S9-S14.
- Parums DV. Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Patients. Med Sci Monit. 2022;28:e935952.
- Zarandi PK, Zinatizadeh MR, Zinatizadeh M, Yousefi MH, Rezaei N. SARS-CoV-2: From the pathogenesis to potential anti-viral treatments. Biomed Pharmacother. 2021;137:111352.
- Hosoki K, Chakraborty A, Sur S. Molecular mechanisms and epidemiology of COVID-19 from an allergist’s perspective. J Allergy Clin Immunol. 2020;146(2):285-299.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:]. Lancet. 2020;395(10223):497-506.
- Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(1):4704.
- Grifoni A, Sidney J, Vita R, et al. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19 [published correction appears in Cell Host Microbe. 2022 Dec 14;30(12):1788]. Cell Host Microbe. 2021;29(7):1076-1092.
- Wallinga J, Teunis P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am J Epidemiol. 2004;160(6):509-516.
- Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063.
- Qu G, Li X, Hu L, Jiang G. An Imperative Need for Research on the Role of Environmental Factors in Transmission of Novel Coronavirus (COVID-19). Environ Sci Technol. 2020;54(7):3730-3732.
- Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W. Incubation Period of COVID-19 Caused by Unique SARS-CoV-2 Strains: A Systematic Review and Meta-analysis [published correction appears in JAMA Netw Open. 2022 Sep 1;5(9):e2235424]. JAMA Netw Open. 2022;5(8):e2228008.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038]. Lancet. 2020;395(10229):1054-1062.
- Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5(12):1598-1607.
- Moreira ED Jr, Kitchin N, Xu X, et al. Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine. N Engl J Med. 2022;386(20):1910-1921.
- Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5):847-859.e11.
- Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227-1230.
- van Riel D, de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19(8):810-812.
- Cai C, Peng Y, Shen E, et al. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol Ther. 2021;29(9):2794-2805.
- Hahn WO, Wiley Z. COVID-19 Vaccines. Infect Dis Clin North Am. 2022;36(2):481-494.
- Nakagami H. Development of COVID-19 vaccines utilizing gene therapy technology. Int Immunol. 2021;33(10):521-527.
- Wallace M, Moulia D, Blain AE, et al. The Advisory Committee on Immunization Practices’ Recommendation for Use of Moderna COVID-19 Vaccine in Adults Aged ≥18 Years and Considerations for Extended Intervals for Administration of Primary Series Doses of mRNA COVID-19 Vaccines – United States, February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(11):416-421.
- Rauch S, Jasny E, Schmidt KE, Petsch B. New Vaccine Technologies to Combat Outbreak Situations. Front Immunol. 2018;9:1963.
- Black S, Eskola J, Siegrist CA, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines [published correction appears in Lancet. 2010 Jan 30;375(9712):376]. Lancet. 2009;374(9707):2115-2122.
- Klein NP, Lewis N, Goddard K, et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA. 2021;326(14):1390-1399.
- Dudley MZ, Halsey NA, Omer SB, et al. The state of vaccine safety science: systematic reviews of the evidence. Lancet Infect Dis. 2020;20(5):e80-e89.
- Rudolph A, Mitchell J, Barrett J, et al. Global safety monitoring of COVID-19 vaccines: how pharmacovigilance rose to the challenge. Ther Adv Drug Saf. 2022;13:20420986221118972.
- Kochhar S, Salmon DA. Planning for COVID-19 vaccines safety surveillance. Vaccine. 2020;38(40):6194-6198.
- Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50(2):279-283.
- Kamidani S, Rostad CA, Anderson EJ. COVID-19 vaccine development: a pediatric perspective. Curr Opin Pediatr. 2021;33(1):144-151.
- Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939-949.
- COVID-19 vaccine safety: the AIFA annual report.
- Dumyati G, Jump RLP, Gaur S. Mandating COVID-19 Vaccine for Nursing Home Staff: An Ethical Obligation. J Am Med Dir Assoc. 2021;22(10):1967-1968.
- Faul F, Erdfelder E Fau – Lang A-G, Lang Ag Fau – Buchner A, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. (1554-351X (Print)).
- https://www.aifa.gov.it/documents/20142/1289678/Comunicato_AIFA_n.627.pdf.
- Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci. 2021;428:117607.
- Läärä E. Statistics: Reasoning on Uncertainty, and the Insignificance of Testing Null. Annales Zoologici Fennici. 2009;46:138-57.
- Kim HY. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod. 2013;38(1):52-4.
- Rapporto sulla Sorveglianza dei vaccini anti-COVID.
- Kislaya I, Machado A, Magalhães S, et al. COVID-19 mRNA vaccine effectiveness (second and first booster dose) against hospitalisation and death during Omicron BA.5 circulation: cohort study based on electronic health records, Portugal, May to July 2022. Euro Surveill. 2022;27(37):2200697.
- Tran Kiem C, Andronico A, Bosetti P, et al. Benefits and risks associated with different uses of the COVID-19 vaccine Vaxzevria: a modelling study, France, May to September 2021. Euro Surveill. 2021;26(26):2100533.
- Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202-221.
- Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245-251.
- Joshi A, Kaur M, Kaur R, Grover A, Nash D, El-Mohandes A. Predictors of COVID-19 Vaccine Acceptance, Intention, and Hesitancy: A Scoping Review. Front Public Health. 2021;9:698111. Published 2021 Aug 13.
- Hudson A, Montelpare WJ. Predictors of Vaccine Hesitancy: Implications for COVID-19 Public Health Messaging. Int J Environ Res Public Health. 2021;18(15):8054. Published 2021 Jul 29.
![]() This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

