Rd. Mustopa1*, Damris2, Syamsurizal2, M. Dwi Wiwik Emawati2
1Department of Medical Laboratory Technology, Health Polytechnic of Jambi, Jambi, Indonesia
2Doctoral Study Program, Faculty of Mathematics and Natural Sciences, Jambi University, Jambi, Indonesia
Corresponding author. Rd. Mustopa, JL. Haji Agus Salim Nomor 09 Kota Baru – Jambi 36361, Indonesia.
Orcid : https://orcid.org/0000-0002-6407-1452.
Phone: +62 821-9668-7959
Email: rdmustopa979@gmail.com
Cite this article
ABSTRACT
Background & Aim: The success of the TB control program is closely related to patient adherence to treatment. Previous studies have provided many views regarding the use of variants of mHealth on TB patient adherence, but the results still need to be clarified. This review aims to evaluate and provide an overview of mHealth RCTs on medication adherence in the patient with tuberculosis.
Methods & Materials: The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline was followed to report study findings. A literature search for studies in the period of 2018-2022 in PubMed, Cochrane, CINAHL and Sciencedirect databases was conducted. Randomized controlled trials (RCTs) that analyzed the effect of mHealth on medication adherence outcomes (treatment completion, treatment adherence, missed doses, and non-completed rate) were included. Adult patients with either active or latent TB infection were included. The Cochrane ’Risk of bias’ assessment tool was used to assess the risk of bias of eligible studies.
Result: Overall, searches on databases generated 2,607 articles, and only 18 articles met the criteria. Two authors independently screened and extracted data from eligible studies. There are two devices used in mHealth in the last five years: software (SMS, We chat, and Whatsapp) and hardware (MERM, eDOT, WOT). Based on descriptive analysis, the hardware mHealth is superior to the software mHealth. Close monitoring and measurement of the use of DOT hardware demonstrates the accuracy of treatment success.
Conclusion: It was found that mHealth interventions can be an advantageous approach. However, the interventions showed variable effects regarding the direction of effect and the rate of improvement of TB treatment adherence and clinical outcomes.
Keywords: Tuberculosis; eHealth; digital health; Adherence; digital adherence.
INTRODUCTION
Tuberculosis is a disease that requires the sufferer’s adherence to a standardized treatment program to completely get rid of Mycobacterium, which is the main cause of this disease, from the sufferer’s body [1–3]. A total of 1.6 million people died from TB in 2021 (including 187,000 people living with HIV). Worldwide, TB is the 13th leading cause of death and the second infectious killer after COVID-19 (above HIV/AIDS). TB is a treatable and curable disease. Drug-susceptible TB disease is treated with a standard 4-month or 6-month course of 4 antimicrobial drugs (isoniazid and rifampicin) that are provided with support to the patient by a health worker or trained treatment supporter [4]. The high number of TB cases worldwide is part of patient non-adherence with treatment programs, which allows for an increase in new TB cases [5]. Non-adherence of TB patients to treatment can be seen from the large number of TB patients who are resistant to standard therapy or what is known as Drug Resistant-Tuberculosis (DR-TB). There are 157,903 Drug Resistant-Tuberculosis (DR-TB) cases in 2020 [6]. To overcome this situation, since 1995 WHO has introduced the DOTs (Directly Observed Treatment, Short-course) strategy. The study states that knowledge is the biggest variable in this aspect of non-adherence, without neglecting other variables such as attitudes and behaviour of TB patients [7]. For this reason, the focus of TB control should be on increasing compliance and changing patient behaviour [8].
The World Health Organization (WHO) has provided a good strategy for managing TB, primarily targeting patient compliance, which has long been known as Directly Observed Treatment (DOT). The strategy consisted of standard treatment using Rifampicin for six months for new cases and eight months for repeat cases [9]. These repeat cases were patients who had dropped out of treatment or failed to undergo previous treatment [10,11]. So, the DOT strategy and program are fine. This strategy requires a better approach and is adapted to the conditions of society. The limitations of the officers who will run this program should be a consideration for the birth of innovations to find which approach is better to do to significantly improve and change the compliance and behaviour of TB patients [12,13]. The birth of a very progressive digital technology that began in the 20th century can be the main choice in solving the problem of treating tuberculosis in the community through innovations in delivering pre-existing programs [14]. In several decades, studies on the use of digital technology to improve TB patient adherence and behaviour have increased sharply in various parts of the world.
The term commonly known today for using mobile devices to support public health care and practice is ‘mHealth, as introduced by WHO. mHealth also includes all mobile devices that use wireless or Bluetooth technology [9]. mHealth is particularly suitable for adherence interventions, as it involves using devices such as smartphones, Personal Digital Assistants (PDAs), tablets and many others [15–18]. These devices support several media, such as Short Messaging Services (SMS) or text messaging, voice or video calls, and specialized software applications (Apps) [15]. Previous studies involving mHealth included Liu and his team, who used a telephone reminder system to increase TB patient compliance [11]. In addition, there are studies using media SMS to serve as reminders for TB patients with good results [19–21].
Based on our initial search of the available studies, the results still need to be clarified. There are no results that show the certainty of the effectiveness of mHealth used. In addition, most of the studies over the five years showed that mHealth variations were similar. Likewise, previous review studies evaluate a lot from just one mHealth variant. To that end, the current review aims to evaluate and provide an overview of mHealth RCTs on medication adherence in the patient with tuberculosis.
METHODS
Design
This review was compiled based on the 2020 Preferred Reporting Items for Systematic-review and Meta-Analysis (PRISMA) guidelines [22].
Eligibility Criteria
This review was restricted to studies published in English, and included studies published through 2018 to 2023. Study types were limited to RCTs. In this review, an intervention for adherence and behaviour were defined as any strategy (e.g., self-management for diseases, and medication reminder) to change or maintain patient’s adherence and behaviour to improve health. We included studies on interventions that used mobile devices (wireless and portable electronics including cellular phones, wearable devices, laptop, personal assistance devices, and tablet PC) or mobile technologies (any technologies that enable communication with remote areas, such as phone call, video call, short messaging service [SMS], multimedia messaging service, online-chat, and email) to promote medication adherence. Observational study, non-intervention study, case report, study protocol, and commentary were excluded in this review.
Information Source
A literature search was performed on several reputable databases, such as PubMed, Sciencedirect, CINAHL, and Cochrane. The search was carried out in the period November 2022 to January 2023.
Search Strategy
The keyword structure was compiled based on study population, intervention, comparison, outcome, and design were developed for the specific databases used. The search strategies for each database provided in the search string table (Table 1).
Selection Process
Two authors independently screened all titles and abstracts from the collected literature. Then read the entire text of each article to assess its eligibility based on predetermined inclusion criteria. Discrepancies that arise are resolved through discussion, even if it is possible to ask for the consideration of the first author. The selection process is described in detail in the PRISMA diagram.
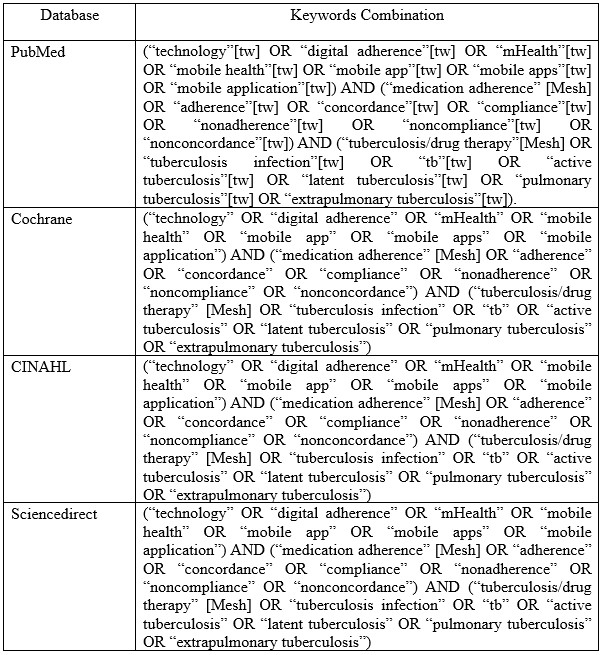
Table 1. Search string in databases
Data Extraction
DM and SR conducted eligibility evaluation based on the title and abstract. The full texts of potentially eligible articles were retrieved and assessed by DM, SR and MD conducted further independent verification of the abstract and full-text screening. Any disagreements among the reviewers were resolved by discussion. Data from the selected articles were extracted by DM, SR, MD and then verified by RM for relevant information, such as publication year, type of mHealth intervention, setting, population, main findings, and control groups.
Assessment of risk of bias in included studies
Two review authors independently assess the risk of bias of each included trial using the Cochrane ’Risk of bias’ assessment tool, and discuss any differences of opinion (Higgins et al., 2011). In the case of missing or unclear information, we will contact the trial authors for clarification. The Cochrane approach assesses risk of bias across six domains: sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential biases. For each domain we will record the methods used by the trial authors to reduce the risk of bias and assign a judgment of either ’low’, ’high’, or ’unclear’ risk of bias.
RESULTS
Overall search on databases resulted in a total of 2,607 articles. After removing 2070 articles for duplication, ineligibility and other reasons, 537 articles were left ready for screening. In the end, 18 articles were declared eligible to be included in this review study after removing 16 articles for reasons including not being an RCT study, not being focused on TB, and being a protocol study.
In full regarding the process of searching for articles can be seen in figure 1, while, in table 2 we reported the characteristics of the articles included in our study
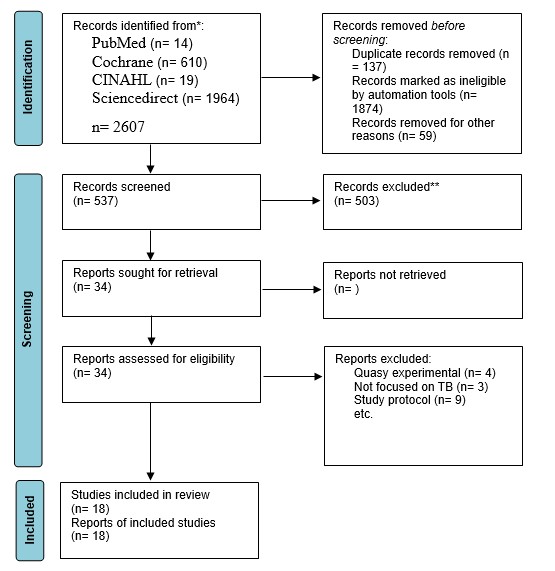
Figure 1. Flow diagram of the studies selection
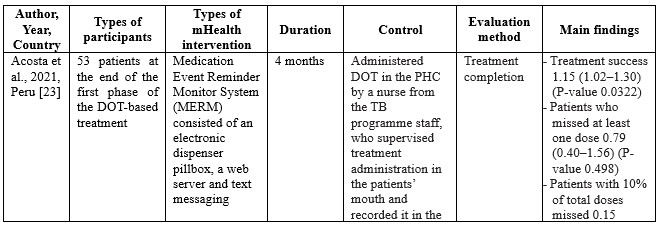

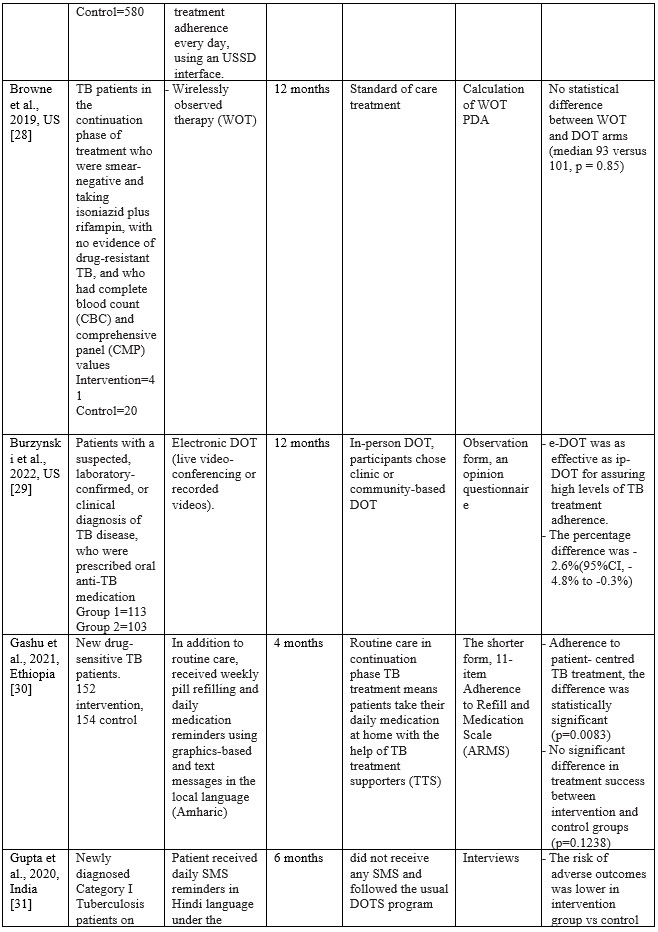
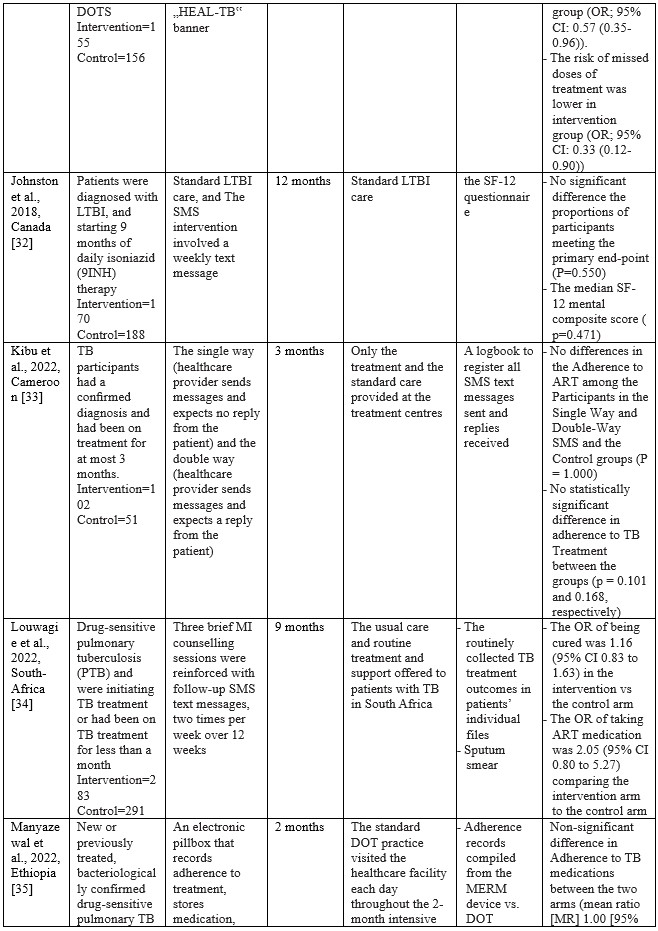
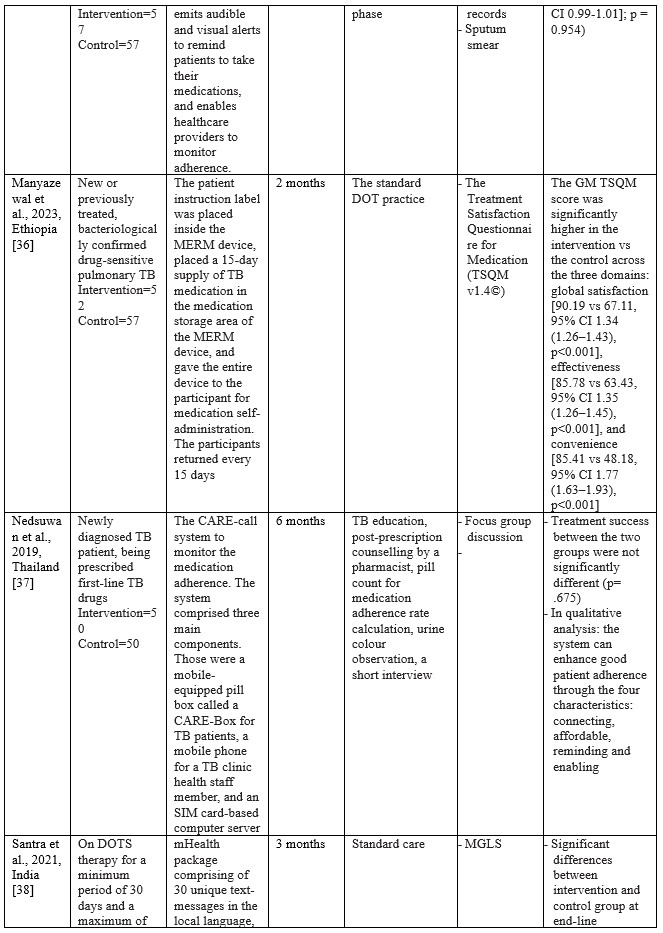

Table 2. Characteristics of Studies Included
Summary of Risk of Bias assessment
The risk of bias in eligible studies using The Cochrane Collaboration’s tool resulted in the conclusion that there were four studies with a high risk of bias and one unclear.
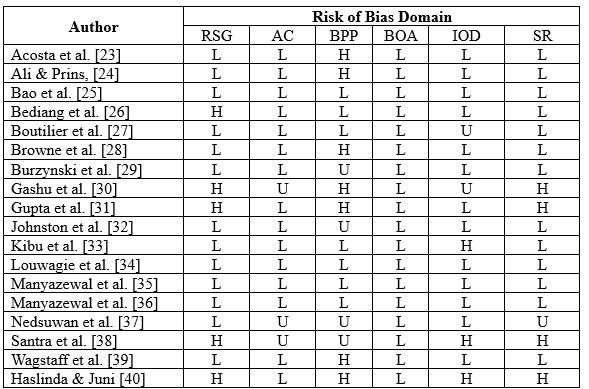
*RSG= Random sequence generation, AC= Allocation Concealment, BPP= Blinding Of Participants and Personnel, BOA= Blinding of Outcome assessment, IOD= Incomplete Outcome Data, SR= Selective Reporting; H= High risk of bias; U= Unclear risk of bias; L= Low risk of bias.
Characteristics of eligible studies
Studies on using m-health applications as innovations to improve adherence, change behaviour, and the success of TB treatment in the last decade have shown a significant increase. We have collected 18 RCT studies from several countries, including Ethiopia (n=3), South Africa (n=2), India (n=2), Cameroon (n=2), US (n=2), and one study each in Thailand, Peru, Sudan, China, Kenya, Canada, and Malaysia. The number of TB patients included in the study ranged from 61 to 1,189, ranging from 18 to 60 years. Most of the studies involved participants newly diagnosed with TB based on positive bacteriology, On DOTS therapy, smears, negative pulmonary tuberculosis (PTB), being prescribed first-line TB drugs, and drug-sensitive pulmonary tuberculosis (PTB). The shortest duration of intervention given was two months, and the longest was 12 months.
m-Health intervention used
Based on the collected studies, the applications used include Short Messages Service (SMS), Medication Event Reminder Monitor System (MERM), WeChat groups, USSD interface, Wirelessly observed therapy (WOT), Digital Adherence Technologies (DATs), Electronic DOT (live video-conferencing or recorded videos), The CARE-call system, and TB@Clicks (Whatsapp).
Several m-Health collected from eligible studies can be broadly grouped into software and hardware applications. In general, m-health applications that use software provide information as reminders and TB education in writing or pictures. Through the SMS route, various interventions are carried out, starting every day, every two days, twice a week, and every week [24,26,27,31–34,38,39]. Through the We-Chat application, there is no time limit for interactions between patients and supervisors taking medication; at any time, patients can discuss all obstacles and questions with supervisors and fellow patients [25]. As for the Whatsapp application, studies report that in the intensive phase, reminders are given to patients every day and 1 to 3 months after the intervention package is carried out [40]. Through telephone calls, patients are also reminded and controlled by supervisors. The duration of each phone call is 10 minutes [24,38].
The hardware used in the intervention includes the Medication Event Reminder Monitor System (MERM), which is a pillbox dispenser that will sound an alarm at the set time to take medicine [23,35,36]. This model is similar to another system called CARE box; it is just that, in this system, when the lid of the box is opened, it will automatically make a missed call to the server [37]. Another device is Wirelessly Observed Therapy (WOT), a sensory device that enters the body to record what the patient consumes, including TB drugs. The data stored on the sensor is linked to a mobile device as information material for supervisors [28]. For E-DOT, a camera device records real-time video of the patient’s medication-taking activities; this system is also used to conduct video conferencing between supervisors and patients [29].
Effects of m-Health on TB patient adherence
In summary, m-Health, with its various variants, has a positive effect in that patients experience increased adherence and changes in behaviour, even though this is not stated explicitly. Several studies have found a positive effect on treatment success related to patient adherence, with P values of 0.0322 [23,24], 0.88 [26], 0.001 [27], 0.85 [28], 0.1238 [30], 0.782 [31], 0.550 [32], 0.101 [33], 0.443 [34], 0.954 [35], 0.001 [36], 0.675 [37], 0.005 [38], 0.03 [40]. Meanwhile, changes in patient behaviour can be seen in findings such as increased self-management behaviour with a P value <0.001 [25], lower risk of missed doses [31], taking ART medication with an OR value 2.05 [34], return to the clinic with a P value of 0.001 [39].
Comparisons between the intervention and control groups in all studies showed no significant differences. However, the intervention using the m-Health variant showed superiority compared to the control group, most of which were in the main form of standard care, Directly Observation Treatment (DOT).
Using MERM, TB patient adherence to treatment is higher than the DOT standard, where TB patients are 1.15 times more compliant when intervened with MERM than the DOT standard [23]. The patients in the SMS intervention group had a lower failure rate (6.8%; 5 of 74 patients) compared to the control group (10.8%; 8 of 74 patients) [24]. In a study conducted by Bediang and colleagues using m-Health in the form of SMS, treatment success was higher in the intervention group compared to the control group (111 patients: 106 patients) [26]. Using SMS messages daily and an unstructured supplementary services data (USSD) interface shows that the probability of unsuccessful treatment outcomes for individuals in the intervention group is approximately 0.08 less than for individuals in the control group [41]. Browne and colleagues found that WOT was superior to DOT in supporting confirmed daily adherence to TB medications, where (3,738 out of 4,022) prescribed doses were confirmed in the WOT treatment, significantly different (p < 0.001) from the 63.1% (1,202 out) of 1,904) of prescribed doses observed in the DOT arm [28]. One hundred seventy-three patients completed the treatment program through the DOT electronic intervention [29]. One hundred ten patients out of a total of 139 TB patients adhered to treatment after intervention using a Mobile phone-based weekly refilling with a daily medication reminder system [30]. Gupta and colleagues found that the treatment success rates in the intervention group using SMS reminders were 86.4%, and the control group was 76.2% [42]. Louwagie and colleagues found that after six months of text SMS intervention, 120 of 133 patients adhered to the TB treatment given [34]. Manyazewal and colleagues using MERM found seven patients completed treatment compared to the control group of 5 [35]. Nedsuwan and colleagues found that using the mobile-based CARE-call system, the number of non-adherence patients in the intervention group was significantly lower than that of the control group (7.5% vs. 27.5%) [37]. Santra and colleagues found that the proportion of participants adherent to DOTS in the intervention group using phone calls and text messages increased from 85.5% at baseline to 96.4% at endline, postintervention [38]. Wagstaff and colleagues found that using SMS messages, as many as 62.0% of patients returned to the clinic in two days compared to 51.5% in the control group [39]. Using the Whatsapp message intervention, Haslinda and Juni found that the number of respondents who adhered to medication was higher in the group that received the intervention (81.8%) compared to the control group (69.1%) [40].
DISCUSSION
This systematic review study aims to evaluate and provide an overview of mHealth RCTs on medication adherence in the patient with tuberculosis which we have successfully conducted by collecting eighteen eligible studies from 2018 to 2022. One of the reasons we limited our literature search to the last five years was to see application innovations that were used along with the development of the digital world in this period. The expectancy is that the latest technological advances in this digitalization era will make it more straightforward to develop information innovations, especially concerning the health sector, to educate patients and the public.
Since the emergence of digital devices, health practitioners are increasingly competing to take advantage of this progress as a good opportunity to help improve public health in preventive and curative ways. M-Health has been attracting attention since it emerged as an innovation that effectively streamlines interactions between healthcare workers and patients, especially in supervising patients such as TB with strict rules for taking drugs for a certain duration. With a relatively lower cost, m-Health can be the first choice in addition to existing programs for monitoring TB patients. For this reason, this study provides an overview of the effectiveness of the m-Health variant from RCT studies in the 2018 to 2022 period regarding adherence and behaviour changes in TB sufferers during the treatment period. The m-Health variants used in the study are software and hardware. This review study analyzed m-Health variants that were not discussed in several previous systematic reviews [43–46].
The m-Health used in the last Five years
Until the last five years, SMS is still an option to remind TB patients to take their medicine. In contrast to previous review studies [45,46], the effectiveness of SMS in monitoring the treatment of TB patients in this review showed no significant difference between the SMS intervention group and the control group with standard care using DOT. Even using the Whatsapp application, TB patient compliance did not show any significance, even though adherence to treatment in the intervention group was higher than the control group [40]. However, with the widespread use of cellphones with the Android system or iPhone Operating System (iOS) among the public, choosing intervention using SMS or chat remains the best choice considering the low cost and efficient application. In contrast to the findings of Bao and colleagues in China, the We-Chat application used as an intervention showed a significant increase in adherence and repeat visits to the clinic during a TB treatment program [25]. Besides the effectiveness of existing smartphone-based applications, various obstacles can be faced, especially for populations in remote areas, where cellular networks and even the internet may be inadequate, especially if the quality of the patient’s cell phone does not support the use of these applications [47].
Behaviours expected of TB sufferers include not spitting, covering the nose and mouth when coughing or sneezing, and wearing a mask [48]. Of course, TB sufferers expect this behaviour to be carried out as one of the steps to prevent the spread of the disease in the surrounding environment [49]. However, the family should be involved in education on the prevention and care of TB patients. The family has an important role in the patient’s treatment process, including preventing the spread of the disease so that it does not affect the people who live in the same house and the people around the house. Families can provide arrangements at home according to good health standards, especially for TB patients. For this reason, further studies need to analyze this educational intervention for families with TB sufferers.
Some of the studies included in this review also provide interventions using a variety of hardware such as the Medication Event Reminder Monitor System (MERM), CARE box, Wirelessly Observed Therapy (WOT), and Electronic-Directly Observation Treatment (E-DOT). These devices are under recommendations from the World Health Organization (WHO) to increase the adherence of TB patients undergoing six months of treatment [14]. Of the six studies that implemented these hardware devices, overall, they showed better success than using software on TB patient adherence to taking medication. The MERM system allows TB patients to take medication daily because the device cover will open at a predetermined time [23]. Manyazewal and the team also used a MERM system with a tool called evriMED500, in the form of a pillbox consisting of a medicine container and an electronic module connected to an indicator light and an alarm [35,36]. The MERM system in the study did not show superiority over the standard care of the control group. However, it should be recognized that the adherence dimension has many independent variables that may play a large role in influencing interventions. Unfortunately, the study of the use of the MERM system that we found did not carry out an analysis of the potential factors. So that bias in the study is likely to occur.
Another device used is Wirelessly Observed Therapy (WOT), a sensory device that enters the body through the mouth. A patch detector in the torso area will read all sensor activity. The data recorded from the patch detector is transmitted wirelessly via Bluetooth technology to mobile phones, computers, or other gadgets [50]. Browne stated that WOT is very safe to apply without significant side effects, only in the form of minimal irritation due to the direct use of patches on the skin [28]. Statistically, WOT is superior to DOT; in other words, WOT is effective in increasing TB patient adherence to treatment. However, the application of WOT is likely to be constrained, especially in countries with lower middle incomes, because this technology is still relatively expensive, and there are suggestions to replace the patch every five days to avoid irritation [28]. Previous studies have also confirmed that using WOT can increase adherence to antiviral HCV therapy in populations at high risk of non-adherence [51].
Another hardware option we found in one study was the use of e-DOT in real-time or recorded video, depending on patient preference [29]. Real-time video allows patients to interact directly with TB program officers with the help of Skype software. Burzynski and colleagues found that e-DOT is similar to in-person-DOT but has equal effectiveness. For this reason, e-DOT can be applied according to the patient’s choice. Especially during a pandemic such as COVID-19, electronic DOT is the best choice to reduce the spread and worsen TB patients’ conditions, as found by Lippincott and colleagues in implementing the Vdot COVID-19 pandemic where this method has high effectiveness and is the first choice. In contrast, in-person DOT is recommended to be carried out later [52]. Haberer and Subbaraman added that implementing eDOT might encounter technical challenges, inaccuracies, costs, and an unsupportive health system [47].
The potential of mHealth on TB patient adherence
Compliance of TB patients with the treatment program can be seen from the success of the treatment. Of the various types of mHealth that we collected, almost all showed an increase in adherence of TB sufferers to the treatment given. Although, comparison with the control group mostly showed insignificant differences.
The use of SMS text generally shows more potential than the DOT standard. Two studies show that compliance with TB patients using SMS text interventions is similar to DOT standards [32,33]. The study states that there may be several factors that influence the failure of TB patient compliance even though they have been reminded via SMS messages, including the lack of more personalized engagement, the didactic nature of the messages, and the SMS message is received when the patient was not near his/her medication all contributed to the failure to reduce poor adherence [53]. For this reason, in the future, this can be a consideration in implementing interventions using text SMS, where controlling these situations is essential to consider. However, based on the success of increasing adherence from studies using text SMS, it was stated that patient compliance was one time greater than the DOT standard. The same thing was also found in the use of Whatsapp, where significant treatment success occurred in TB patients who were given education through messages via Whatsapp [40].
The medication event reminder monitor (MERM) system in studies using it also shows positive potential to improve TB patient adherence to treatment. In addition, using MERM can also reduce the workload of health workers [54]. One problem identified using MERM is the possibility of removal of the medication from the pillbox, for example, for work-related reasons, which prevents the recording of pill dispensing. Although the potential of MERM is not superior to in-person DOT, MERM can be used as an alternative to improve TB patient compliance. The identical thing is also found in using electronic DOT and WOT. This hardware allows stricter supervision and accurate recording of each drug-taking activity so that health workers can more easily measure treatment success.
LIMITATION
The limitations encountered in this review include limited access to several reputable databases, which does not allow us to explore further relevant articles. In addition, this review includes studies of low to high quality due to the small number of articles we have collected. For this reason, writers who want to use the results of this review must be careful and analyze them more carefully.
CONCLUSION
This review shows that using m-Health can be the first choice in handling TB cases with the DOT strategy. Hardware as part of mHealth has more potential to increase TB patient adherence and behaviour change. TB patient compliance with medication programs and stopping the spread of TB through good behaviour will be very significant in reducing TB cases, recurrent cases and new cases. mHealth is the best choice as a companion to the ongoing DOT program, primarily as a medium for disseminating information needed by patients during their treatment period. In the era of digitalization today and in the future, mHealth is undoubtedly the main route in health services, as illustrated during the pandemic of certain diseases that did not allow face-to-face meetings. However, further efficacy studies at the clinical level are needed, while always protecting privacy.
REFERENCES
- Acharya B, Acharya A, Gautam S, Ghimire SP, Mishra G, Parajuli N, et al. Advances in diagnosis of Tuberculosis: an update into molecular diagnosis of Mycobacterium tuberculosis. Molecular biology reports. 2020;47:4065–75.
- Asriati A, Kusnan, Adius, Alifariki L. Faktor Risiko Efek Samping Obat dan Merasa Sehat Terhadap Ketidakpatuhan Pengobatan Penderita Tuberkulosis Paru. JURNAL KESEHATAN PERINTIS (Perintis’s Health Journal). 2019;6(2):134–9.
- Putri S, Alifariki LO, Fitriani F, Mubarak M. The Role of Medication Observer And Compliance In Medication Of Pulmonary Tuberculosis Patient. Jurnal Kesehatan Prima [Internet]. 2020 Feb 29;14(1). Available from: http://jkp.poltekkes-mataram.ac.id/index.php/home/article/view/248
- World Health Organization (WHO). Tuberculosis [Internet]. 27 October 2022. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- Harding E. WHO global progress report on tuberculosis elimination. The Lancet Respiratory Medicine. 2020;8(1):19.
- Dean AS, Auguet OT, Glaziou P, Zignol M, Ismail N, Kasaeva T, et al. 25 years of surveillance of drug-resistant tuberculosis: achievements, challenges, and way forward. The Lancet Infectious Diseases. 2022;
- Ghozali MT, Murani CT. Relationship between knowledge and medication adherence among patients with tuberculosis: a cross-sectional survey. Bali Medical Journal. 2023;12(1):158–63.
- Asriati A. Faktor Risiko Ketidakpatuhan Pengobatan Penderita Tuberkulosis Paru di Kota Kendari. Jurnal Keperawatan Terapan (e-Journal). 2019;5(2):103–10.
- World Health Organization. Digital health for the End TB Strategy: an agenda for action. World Health Organization; 2015.
- El Emeiry F, Shalaby S, El-Magd GHA, Madi M. Treatment outcomes of tuberculosis among new smear-positive and retreatment cases: a retrospective study in two Egyptian governorates. The Egyptian Journal of Chest Diseases and Tuberculosis. 2019;68(3):274.
- Ying R, Huang X, Gao Y, Wang J, Liu Y, Sha W, et al. In vitro Synergism of Six Antituberculosis Agents Against Drug-Resistant Mycobacterium tuberculosis Isolated from Retreatment Tuberculosis Patients. Infection and Drug Resistance. 2021;3729–36.
- Margineanu I, Louka C, Vincenti-Gonzalez M, Saktiawati AMI, Schierle J, Abass KM, et al. Patients and medical staff attitudes toward the future inclusion of ehealth in tuberculosis management: perspectives from six countries evaluated using a qualitative framework. JMIR mHealth and uHealth. 2020;8(11):e18156.
- Zhang J, Yang Y, Qiao X, Wang L, Bai J, Yangchen T, et al. Factors influencing medication nonadherence to pulmonary tuberculosis treatment in tibet, china: a qualitative study from the patient perspective. Patient preference and adherence. 2020;1149–58.
- World Health Organization. Handbook for the use of digital technologies to support tuberculosis medication adherence. World Health Organization; 2017.
- Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. Journal of medical Internet research. 2015;17(2):e52.
- Bartels SL, Van Knippenberg RJM, Dassen FCM, Asaba E, Patomella A-H, Malinowsky C, et al. A narrative synthesis systematic review of digital self-monitoring interventions for middle-aged and older adults. Internet interventions. 2019;18:100283.
- He Q, Zhao X, Wang Y, Xie Q, Cheng L. Effectiveness of smartphone application–based self‐management interventions in patients with type 2 diabetes: A systematic review and meta‐analysis of randomized controlled trials. Journal of advanced nursing. 2022;78(2):348–62.
- Zhu MM, Choy BNK, Lam WWT, Shum J. Randomized control trial of the impact of Patient Decision Aid (PDA) developed for Chinese primary open-angle glaucoma patients. Ophthalmic Research. 2023;
- Hirsch-Moverman Y, Daftary A, Yuengling KA, Saito S, Ntoane M, Frederix K, et al. Using mHealth for HIV/TB treatment support in Lesotho: enhancing patient–provider communication in the START study. Journal of acquired immune deficiency syndromes (1999). 2017;74(Suppl 1):S37.
- Mohammed S, Glennerster R, Khan AJ. Impact of a daily SMS medication reminder system on tuberculosis treatment outcomes: a randomized controlled trial. PloS one. 2016;11(11):e0162944.
- Farooqi RJ, Ahmed H, Ashraf S, Zaman M, Farooq S, Farooqi JI. Feasibility and acceptability of Mobile SMS reminders as a Strategy to improve drugs adherence in TB Patietns. Pakistan Journal of Chest Medicine. 2017;23(3):93–100.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. International Journal of Surgery. 2021;88:105906.
- Acosta J, Flores P, Alarcon M, Grande-Ortiz M, Moreno-Exebio L, Puyen ZM. A randomised controlled trial to evaluate a medication monitoring system for TB treatment. The International Journal of Tuberculosis and Lung Disease. 2022;26(1):44–9.
- Ali AOA, Prins MH. Mobile health to improve adherence to tuberculosis treatment in Khartoum state, Sudan. Journal of Public Health in Africa. 2019;10(2).
- Bao Y, Wang C, Xu H, Lai Y, Yan Y, Ma Y, et al. Effects of an mHealth intervention for pulmonary tuberculosis self-management based on the integrated theory of health behavior change: randomized controlled trial. JMIR public health and surveillance. 2022;8(7):e34277.
- Bediang G, Stoll B, Elia N, Abena J-L, Geissbuhler A. SMS reminders to improve adherence and cure of tuberculosis patients in Cameroon (TB-SMS Cameroon): a randomised controlled trial. BMC public health. 2018;18:1–14.
- Boutilier JJ, Yoeli E, Rathauser J, Owiti P, Subbaraman R, Jónasson JO. Can digital adherence technologies reduce inequity in tuberculosis treatment success? Evidence from a randomised controlled trial. BMJ Global Health. 2022;7(12):e010512.
- Browne SH, Umlauf A, Tucker AJ, Low J, Moser K, Gonzalez Garcia J, et al. Wirelessly observed therapy compared to directly observed therapy to confirm and support tuberculosis treatment adherence: a randomized controlled trial. PLoS medicine. 2019;16(10):e1002891.
- Burzynski J, Mangan JM, Lam CK, Macaraig M, Salerno MM, deCastro BR, et al. In-person vs electronic directly observed therapy for tuberculosis treatment adherence: A randomized noninferiority trial. JAMA Network Open. 2022;5(1):e2144210–e2144210.
- Gashu KD, Gelaye KA, Lester R, Tilahun B. Effect of a phone reminder system on patient-centered tuberculosis treatment adherence among adults in Northwest Ethiopia: a randomised controlled trial. BMJ Health & Care Informatics. 2021;28(1).
- Gupta A, Bhardwaj AK, Singh H, Kumar S, Gupta R. Effect of ‘mHealth’Interventions on adherence to treatment and outcomes in Tuberculosis patients of district Shimla, Himachal Pradesh, India: A Randomised Control Trial. Indian Journal of Preventive & Social Medicine. 2020;51(3):125–36.
- Johnston JC, van der Kop ML, Smillie K, Ogilvie G, Marra F, Sadatsafavi M, et al. The effect of text messaging on latent tuberculosis treatment adherence: a randomised controlled trial. European Respiratory Journal. 2018;51(2).
- Kibu OD, Siysi VV, Albert Legrand SE, Asangbeng Tanue E, Nsagha DS. Treatment Adherence among HIV and TB Patients Using Single and Double Way Mobile Phone Text Messages: A Randomized Controlled Trial. Journal of Tropical Medicine. 2022;2022.
- Louwagie G, Kanaan M, Morojele NK, Van Zyl A, Moriarty AS, Li J, et al. Effect of a brief motivational interview and text message intervention targeting tobacco smoking, alcohol use and medication adherence to improve tuberculosis treatment outcomes in adult patients with tuberculosis: a multicentre, randomised controlled tri. BMJ open. 2022;12(2):e056496.
- Manyazewal T, Woldeamanuel Y, Holland DP, Fekadu A, Marconi VC. Effectiveness of a digital medication event reminder and monitor device for patients with tuberculosis (SELFTB): a multicenter randomized controlled trial. BMC medicine. 2022;20(1):310.
- Manyazewal T, Woldeamanuel Y, Getinet T, Hoover A, Bobosha K, Fuad O, et al. Patient-reported usability and satisfaction with electronic medication event reminder and monitor device for tuberculosis: a multicentre, randomised controlled trial. eClinicalMedicine. 2023;56:101820.
- Ratchakit-Nedsuwan R, Nedsuwan S, Sawadna V, Chaiyasirinroje B, Bupachat S, Ngamwithayapong-Yanai J, et al. Ensuring tuberculosis treatment adherence with a mobile-based CARE-call system in Thailand: a pilot study. Infectious Diseases. 2020;52(2):121–9.
- Santra S, Garg S, Basu S, Sharma N, Singh MM, Khanna A. The effect of a mhealth intervention on anti-tuberculosis medication adherence in Delhi, India: A quasi-experimental study. Indian Journal of Public Health. 2021;65(1):34.
- Wagstaff A, Van Doorslaer E, Burger R. SMS nudges as a tool to reduce tuberculosis treatment delay and pretreatment loss to follow-up. A randomized controlled trial. PLoS One. 2019;14(6):e0218527.
- Haslinda N, Juni MH. Effectiveness of health education module delivered through Whatsapp to enhance treatment adherence and successful outcome of tuberculosis in Seremban district, Negeri Sembilan, Malaysia. International Journal of Public Health and Clinical Sciences. 2019;6(4):145–59.
- Boutilier JJ, Jónasson JO, Yoeli E. Improving tuberculosis treatment adherence support: the case for targeted behavioral interventions. Manufacturing & Service Operations Management. 2022;24(6):2925–43.
- Das Gupta D, Patel A, Saxena D, Koizumi N, Trivedi P, Patel K, et al. Choice‐Based Reminder Cues: Findings From an mHealth Study to Improve Tuberculosis (TB) Treatment Adherence Among the Urban Poor in India. World Medical & Health Policy. 2020;12(2):163–81.
- Laksono EB, Johan A, Erawati M. The Utilization of Mobile-Health Intervention In Improving Treatment Compliance Behavior In Tuberculosis Patients. Nurse and Health: Jurnal Keperawatan. 2022;11(2):275–86.
- Shariful IM. Theories applied to m-health interventions for behavior change in low-and middle-income countries: a systematic review. Telemedicine and e-Health. 2018;
- Nglazi MD, Bekker L-G, Wood R, Hussey GD, Wiysonge CS. Mobile phone text messaging for promoting adherence to anti-tuberculosis treatment: a systematic review. BMC infectious diseases. 2013;13(1):1–16.
- Sarabi RE, Sadoughi F, Orak RJ, Bahaadinbeigy K. The effectiveness of mobile phone text messaging in improving medication adherence for patients with chronic diseases: a systematic review. Iranian Red Crescent Medical Journal. 2016;18(5).
- Haberer JE, Subbaraman R. Digital technology for tuberculosis medication adherence: promise and peril. Vol. 17, Annals of the American Thoracic Society. American Thoracic Society; 2020. p. 421–3.
- Lucya V. Prevention of Tuberculosis: Literature Review. KnE Life Sciences. 2021;630–4.
- Lönnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. The lancet Diabetes & endocrinology. 2014;2(9):730–9.
- Hafezi H, Robertson TL, Moon GD, Au-Yeung K-Y, Zdeblick MJ, Savage GM. An ingestible sensor for measuring medication adherence. IEEE Transactions on Biomedical Engineering. 2014;62(1):99–109.
- Bonacini M, Kim Y, Pitney C, McKoin L, Tran M, Landis C. Wirelessly observed therapy to optimize adherence and target interventions for oral hepatitis C treatment: observational pilot study. Journal of Medical Internet Research. 2020;22(4):e15532.
- Lippincott CK, Perry A, Munk E, Maltas G, Shah M. Tuberculosis treatment adherence in the era of COVID-19. BMC infectious diseases. 2022;22(1):800.
- Liu X, Lewis JJ, Zhang H, Lu W, Zhang S, Zheng G, et al. Effectiveness of electronic reminders to improve medication adherence in tuberculosis patients: a cluster-randomised trial. PLoS medicine. 2015;12(9):e1001876.
- Wang N, Zhang H, Zhou Y, Jiang H, Dai B, Sun M, et al. Using electronic medication monitoring to guide differential management of tuberculosis patients at the community level in China. BMC infectious diseases. 2019;19(1):1–9.
![]() This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

