Herinawati1*, Atikah Fadhilah Danaz Nasution1, Lia Artika Sari1, Iksaruddin2
1Midwifery Department, Health Polytechnic of the Ministry of Health, 36128 Jambi, Indonesia
2Department of Health Promotion, Health Polytechnic of Jambi, 36128, Indonesia
Corresponding Author: Herinawati, herinawati.poltekkes@gmail.com, Jl. Prof DR GA Siwabessy No.42, Buluran Kenali, Kec. Telanaipura, Kota Jambi, 36122
Cita questo articolo
ABSTRACT
Background: The inappropriate temperature of vaccine storage may cause vaccine damage leading to degrading or even dispelling the vaccine’s quality. This research aims to reveal the difference of vaccine’s quality stored in the cooler box compared to the household refrigerator with vaccine only and the difference of vaccine’s quality stored in the cooler box compared to the household refrigerator with vaccine stored along with food and beverage
Methods: The research design uses an experimental study with a post-test-only control group design. The research was conducted at two independent practice midwives in Antapani Sub-district and the Faculty of Mechanical and Aerospace Engineering Bandung Institute of Technology from November to December 2015. The research object used eight types of vaccine (Hepatitis B, BCG, DPT-HB-Hib, Polio, Measles, DT, Td, and TT) with 72 vaccine vials divided into 3 vaccine storage units. The data analysis uses the chi-square test.
Results: The results of the research show that there are statistically significant differences between the vaccine’s quality stored using a cooler box compared to a household refrigerator with vaccine only and the vaccine’s quality stored using a cooler box compared to a household refrigerator with vaccine stored along with food and beverage with the value of p<0,05.
Conclusion: It is concluded that there are differences of the vaccine’s quality stored using cooler box compared to a household refrigerator with vaccine only and also different vaccine’s quality stored using cooler box compared to a household refrigerator with vaccine stored along with food and beverage
Keywords: Cooler box, household refrigerator, vaccine quality
INTRODUCTION
Vaccines are biological preparations to increase immunity against several diseases[1]. Immunization will be effective if the vaccine is distributed evenly with well-maintained quality. The quality of the vaccine is influenced by the time of distribution because the life of the vaccine is very limited and requires special treatment. For this reason, the cold chain must be adequate as a guarantee of vaccine quality [2]. The vaccine cold chain system is a series of storage and transportation processes using various equipment accordingly to ensure the quality of the vaccine from the factory to the patient [3].
The Minister of Health Regulation Number 12 of 2017 concerning the Implementation of Immunization has standardized a vaccine storage temperature of 2 to 8ºC for freeze sensitive vaccines (not frozen), and at a temperature of -15 to -25 C for heat sensitive vaccines. Currently, only polio vaccine still requires storage at temperatures below 0°C. A number of vaccines, such as Hepatitis B, DPT-HB-Hib, IPV, DT, Td will potentially be damaged if exposed to freezing temperatures. Meanwhile, the Polio, BCG, and Measles vaccines will potentially be damaged if exposed to hot temperatures. However, in general, vaccines will spoil if exposed to direct sunlight. In general, the cold chain consists of refrigerators and freezers to store vaccines, and thermos (vaccine carriers) to bring vaccines to immunization services, especially for activities outside the building/field [4]. Annually, more than 1.4 million children in the world die from various diseases, which should be avoided by immunization. Several infectious diseases that are included in the Disease Preventable by Immunization include: diphtheria, tetanus, hepatitis B, meningitis, pneumonia, polio, pertussis, and measles. Children who have been immunized will be protected from these dangerous diseases, which can cause disability or death[5,6]. Immunization for infants is called basic immunization, while immunization for primary school-age children and women of childbearing age is called advanced immunization. Vaccines for routine immunization in infants include: hepatitis B, BCG, polio, DPT, and measles. At school age: Diphtheria Tetanus (DT), Diphtheria Tetanus (Td). Immunization of women of childbearing age is given tetanus toxoid[7]. Incompatibility of the vaccine storage temperature with the standard, resulting in damage to the vaccine which means lowering the quality of the vaccine [8].Vaccine quality cannot be improved even if it is stored again at the right temperature. Defective vaccines must be destroyed, because it cannot induce immunity in the body through immunization, in fact itmayaffect Post Immunization Adverse Events (AEFI) to the target. Household refrigerators are designed to store food and drink, it is not recommended to store vaccines. At the district and immunization service units, most household refrigerators are used to store vaccines. Research conducted in Tunisia on 10 household refrigerators found that many household refrigerators found problems with freezing and high temperatures, making it a risk for vaccine storage[9]. Cooler box, a new tool made by ITB students, is an innovation in the medical field that utilizes technology to store vaccines, uses electricity and has a stable temperature (+4.63oC)[10]. Cooler Box’s ability to store vaccines is measured through a vaccine vial monitor (VVM). All vaccines in the immunization program are equipped with VVM, which is an indicator attached to each vaccine vial to monitor vaccines during transit and storage. The combined effects of time and temperature provide information about heat exposure and potency.
In Indonesia, there are still a number of deaths from Immunization Preventable Diseases (PD3I), including tuberculosis (TB) and measles [11]. In 2013 West Java Province achieved the highest measles immunization coverage in Indonesia at 95.8%, with the incidence of measles at 1,910 cases, occurring in the immunized group as many as 562 cases (34%). Measles Extraordinary Events (KLB) occurred in West Java as many as 18 outbreaks with 205 cases [11]. Another disease that is included in PD3I, and is still high in West Java is tuberculosis with a total of 61,721 people (306 patients aged <12 years)[11]. The city of Bandung is the center of the province of West Java and has 73 health centers (30 UPTs and 34 networks). In 2013 UPT Griya Antapani had 31 cases of measles and increased in 2014 to 33 cases of measles. Other diseases that are included in PD3I are at UPT Griya Antapani, namely tuberculosis [11].
The success of the immunization program cannot be determined only by the high coverage rate, but also by the reduction in cases and outbreaks of PD3I as an indicator of increasing service quality. [12]. Several studies have revealed that outbreaks can be caused by low immunization coverage or due to low vaccine quality caused by poor vaccine cold chain management [13,14].
Based on the existing problems, we tested a tool that had been developed by ITB students and was an innovation for storing vaccines. Trials by storing vaccines for 4 weeks in cooler boxes, household refrigerators filled with vaccines, and household refrigerators filled with vaccines with food and drinks. Furthermore, a vaccine vial monitor (VVM) was assessed to determine the quality of the vaccine.
METHODS
This experiment uses the Post test only control group design approach. The research group consisted of two groups including vaccines stored in household refrigerators and vaccines stored in cooler boxes. The study was conducted at 2 independent midwife clinics in Antapani sub-district and the FTMD ITB laboratory from November to December 2015. The object of the study used 8 types of vaccines (Hepatitis B, BCG, DPT-HB-Hib, polio, measles, DT, Td and TT) with a total sample of 72 vaccine vials which were divided into 3 vaccine storage devices: cooler box, refrigerator filled only with vaccines and household refrigerator filled with food and drinks.
Collecting research data by recording the temperature of household refrigerators and refrigerators, researchers make visits to the midwife’s clinic 2 times a day, in the morning at 09.00-11.00 and in the afternoon at 17.00-19.00. Recording the cooler box temperature in the FTMD ITB laboratory, in the morning at 8.00-10.00 and in the afternoon at 15.00-17.00 after one month of vaccine quality assessment (VVM). The temperature of the cooler box and refrigerator is set (+20C)-(+8oC).
The limits for the condition indicators of the vial monitor vaccine (VVM) are detailed in the following Table 1.

Table 1. Various vaccine vial monitor (VVM) conditions
The study was performed in accordance with the ethical considerations of the Helsinki Declaration. The Health Research Ethics Commission of the Faculty of Medicine, Padjadjaran University has given clearance approval with certificate number 694/UN6.C1.3.2/KEPK/PN2015. The ethical aspects of this study consider in detail the place of research to be used.
Statistical analysis
Data are presented as numbers and percentages for categorical variables. Continuous data are expressed as the mean ± standard deviation (SD), or median with Interquartile Range (IQR). In the case of categorical variables, the chi-square test is performed to test the difference between the two independent samples. All tests with p-value (p) <0.05 were considered significant. Statistical analysis was performed using SPSS app version 16.0.
RESULTS
The 8 types of vaccines used include Hepatitis B, BCG, polio, DPT-HB-Hib, measles, TT, DT and Td. Vaccines are stored for one month using three vaccine storage devices which are placed in different places, including a cooler box in the FTMD ITB laboratory, a household refrigerator and a refrigerator containing only vaccines at the midwife’s clinic. The results showed a statistically significant difference in vaccine quality (VVM) using a cooler box compared to a household refrigerator containing vaccines, food, and beverages (p<0.05) (Table 2).
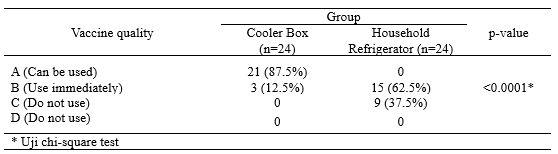
Table 2. Differences in the Quality of Vaccines stored in Cooler Boxes Compared to Household Refrigerators
The results also showed a statistically significant difference in the quality of the vaccine (VVM) stored in a cooler box compared to a refrigerator containing only vaccine (p<0.05) (Table 3).
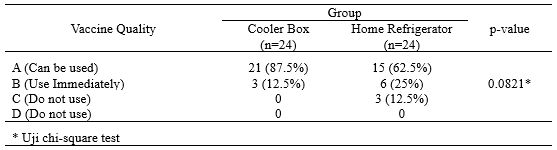
Table 3. Differences in the Quality of Vaccines Stored in Cooler Boxes Compared to Home Refrigerators containing Vaccines
The temperature fluctuations in the cooler box during the morning and afternoon inspections can be seen in Figure 1. Descriptively there is a difference in the temperature of the cooler box, where in the afternoon it is higher than in the morning. up to 7.30C.
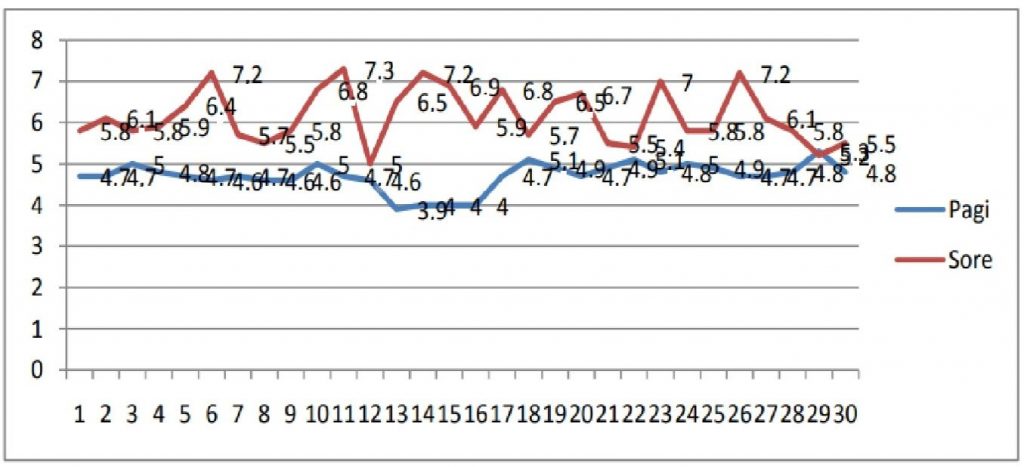
Figure 1. Vaccine Temperature Chart Stored in Cooler Box
The temperature fluctuations in the household refrigerator during the morning and afternoon inspection can be seen in Figure 2. Descriptively there is a difference in the temperature of the household refrigerator, which is higher in the afternoon than in the morning. The highest household refrigerator temperature in the morning is 11.7oC and in the afternoon it reaches 18.1oC.
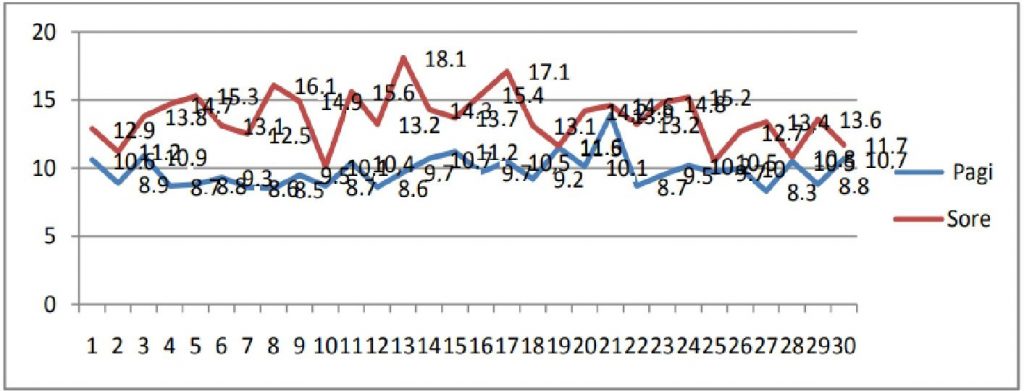
Figure 2. Graph of Vaccine Temperature Stored in Household Refrigerators
Temperature fluctuations in the refrigerator containing only vaccines in the morning and afternoon examinations can be seen in Figure 3, where descriptively there is a difference in temperature in the refrigerator containing only vaccines, which is higher in the afternoon than in the morning. The temperature of the refrigerator containing the vaccine only in the morning is 7.1oC and in the afternoon it reaches 13.1oC.
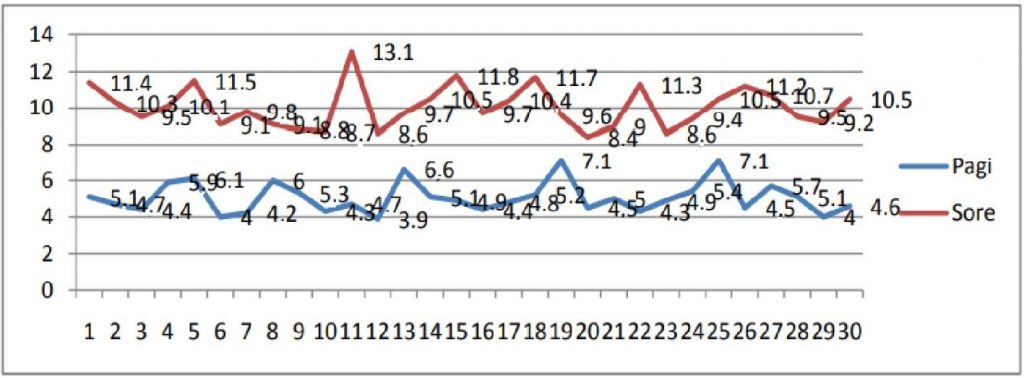
Figure 3. Vaccine Temperature Chart Stored in the Refrigerator Only Contains Vaccine
DISCUSSIONS
Vaccines have certain characteristics and require a special cold chain since they are produced in the factory until they are used in health care units. Deviations from the storage of vaccines from existing provisions can result in damage to the vaccine so that it reduces or even eliminates the quality of the vaccine, resulting in no immunity. Monitoring of vaccine storage temperatures is crucial in establishing vaccine viability. Vaccine is a biological product that is sensitive to temperature, exposure to heat will shorten the shelf life of the vaccine. Vaccine quality depends on the cold chain, with a defined temperature range from (+2oC)-(+8oC) during transport and storage [7]. Vaccine vial monitors (VVM) are used to assess whether the vaccine has ever been exposed to temperatures above the allowable limits, it is said to be VVM A or VVM B conditions. must be used immediately. The condition of VVM C or VVM D if the color of the rectangular box is the same or darker than the circle and its surroundings, then the vaccine has been exposed to temperatures above the permissible limit, the vaccine should not be used.
The results of this study revealed a significant difference in the quality of vaccines (VVM) stored in cooler boxes compared to household refrigerators (p=0.001). WHO and the Indonesian Ministry of Health do not recommend household refrigerators for vaccine storage because they are not designed to maintain a temperature (+2oC)-(+8oC) as the temperature range used to store vaccines, this is due to the rapid changes in warm temperatures when fridge door opened [15].
In accordance with the recommendations of WHO and the Indonesian Ministry of Health, it is not allowed to store food, drinks, medicines, or other objects in the vaccine refrigerator, it will disrupt temperature stability because it is often opened Any refrigerator or freezer used for vaccine storage should be dedicated only to vaccine storage without any food and drink mixes. The results of trials conducted by WHO, showed that a refrigerator or freezer for storing vaccines that is often opened can cause the refrigerator temperature to be unstable because warm air flows into the refrigerator every time the refrigerator door is opened and results in food and beverage spills and contamination [6,16]. WHO and the Indonesian Ministry of Health have recommended monitoring the temperature of the vaccine storage area twice a day, in the morning and evening before taking the vaccine. The refrigerator door should not be opened for more than one minute at a time when the vaccine is taken (avoid opening the refrigerator door too often as possible). Research conducted by WHO shows that frequently opening the refrigerator door can cause temperature instability and excessive exposure to light [15]. Several studies have found poor knowledge and practice in administering immunization vaccines in primary care centers, also found a lack of attitudes and behavior of midwives in administering vaccines according to established standards. The behavior of midwives in vaccine management is strongly influenced by the level of knowledge of the midwife about vaccines and their management. The quality of immunization is closely related to how the vaccine is handled and treated, and the maintenance of the cold chain. Vaccine management is part of the quality of service. Indicators of good vaccine management quality are indicated by maintained vaccine temperature (+2)-(+8)oC, no vaccine found with VVM C or VVM D[17]. Research in Bandung City showed that midwives had low knowledge of vaccine storage, some midwives showed a negative attitude towards vaccine storage and most respondents did not practice vaccine storage according to standards. Research in the city of Bogor found that most of the vaccines stored in the midwife clinic at the same time as placing food such as fruits, cakes, eggs, meat and vegetables, and beverages, and it was found that most of the vaccines were in VVM C and VVM D[18]. The current study found that the vaccine quality in the cooler box was better than the household refrigerator containing only vaccine (p<0.05) where there was a significant difference, this was related to the temperature in the cooler box where the vaccine was stored according to the standard (+2oC)-(+8oC) while the household refrigerator filled with vaccines did not meet the standard, the temperature (> 8oC) was found in the afternoon recording. These results are consistent with several previous studies, such as in Kelantan Malaysia found 73.5% of household refrigerators found that the temperature was more than 8oC.17 Studies in Thailand and Cameroon also showed high warm temperatures in vaccine storage using household refrigerators[19,20], and a study in Semarang City found that 52.2% of household refrigerator temperatures had a temperature > 8oC [21]. Thus, the results of the current study are in line with previous studies where the results of household refrigerator temperatures are more than 8oC. Several things related to higher refrigerator temperatures or unstable refrigerator temperatures, including the refrigerator door not closing tightly, this usually occurs because the rubber door on the refrigerator door is uneven or torn so that the cold air inside the refrigerator exchanges with air outside the refrigerator. As a result, low temperatures in the refrigerator are difficult to achieve or there is a refrigerant or freon leak in the refrigerant system which results in disruption of the cooling process because there is no refrigerant as a medium for heat transfer from inside the refrigerator to outside the refrigerator [16]. These results indicate that the polio vaccine stored in the cooler box is in VVM B (the vaccine is immediately used) while in the household refrigerator the contents of the vaccine are in VVM C [22]. Polio vaccine is stable for 6 months if stored at (+2oC)-(+8oC), and stable for 2 years if stored at (-15oC)-(-25oC). The polio vaccine does not contain preservatives, stabilizers, and adjuvants. Preservatives are used to prevent bacterial and/or fungal contamination (contamination) of bacteria and/or fungi into vaccines. Stabilizers are added in vaccine production to ensure vaccines are subject to extreme conditions or changing environmental conditions, such as heat, light, humidity, and acidity. Adjuvant are substances used to enhance the immune response of a vaccine, optimize immune system-stimulating cells, reduce the number of antigens used in a vaccine and reduce the frequency of administration [23].
The BCG vaccine is derived from live attenuated bacteria and the measles vaccine is derived from a live attenuated virus. Storage of BCG vaccine at a temperature (-15oC)-(-25oC)or at a temperature (+2oC)-(+8oC) there is no difference, BCG vaccine is stable for 1 year. Measles vaccine is derived from live attenuated virus, measles vaccine is stored at (-15oC)-(-25oC)or at (+2oC)-(+8oC) temperature there is no difference, measles vaccine is stable for 2 years[6].
Similar to polio vaccine, BCG vaccine and measles vaccine do not contain preservatives, stabilizers and adjuvants. Preservatives are used to prevent bacterial and/or fungal contamination (contamination) of vaccines. Stabilizers are added in vaccine production to ensure vaccines are subjected to extreme conditions or changing environmental conditions, such as heat, light, humidity, and acidity[24]. Adjuvants are substances used to enhance the immune response of a vaccine, optimize immune system-stimulating cells, reduce the number of antigens used in a vaccine and reduce the frequency of administration[25]. In addition, the vaccine is also light sensitive and the tinted glass vial has been shown to minimize potency loss[26].
The current study revealed that high temperatures (>8oC) in vaccine storage using household refrigerators containing only vaccines can cause changes in VVM in polio, BCG, and measles vaccines. The polio vaccine was obtained with VVM C while the BCG and measles vaccines with VVM B this was related to the use of VVM 2 in the polio vaccine (low stability) while the BCG vaccine and measles vaccine used VVM 14 (medium stability)[5].
In this study, it was found that there was no difference between the quality of the vaccine stored in the cooler box and the home refrigerator containing the vaccine because the quality of the vaccine was safe and maintained in the refrigerator. The reason is that the home refrigerator containing the vaccine is not opened often, and this is what distinguishes the vaccine from being stored in a refrigerator containing food ingredients, because it is often opened so that the temperature in the refrigerator becomes high or more than 8oC.
Based on research at the National Institute of Standards and Technology (NIST) Gaithersburg Thermal Studies Laboratory found a way to maintain cold temperatures in household refrigerators that lose cold temperatures due to frequent openings or as a result of lights, namely inserting ice bottles in household refrigerators which has an impact on prolonging time cold in the fridge. Thermal ballast usage is a practical and effective strategy for mitigating the negative impact of power outage events[27].
CONCLUSIONS
There is a difference in the quality of vaccines stored in cooler boxes compared to household refrigerators. There is a difference in quality that is stored in a cooler box compared to a refrigerator containing only vaccines. Finally, the correct cold chain for the good conservation of vaccines must always be maintained according to the indications of the manufacturers
LIMITATION OF STUDY
The limitation of this study is the small sample size
CONFLICT OF INTEREST
The authors have no conflict of interest.
FUNDING STATEMENT
The author(s) received no financial support for the research, authorship, and/or publication of this article.
REFERENCES
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nature Reviews Immunology [Internet]. 2021;21(2):83–100. Available from: https://doi.org/10.1038/s41577-020-00479-7
- Yuniar Y, Syaripuddin M, Sasanti R, Susyanty AL. Sistem Manajemen dan Persediaan Vaksin di Dua Provinsi Indonesia. Indonesian Bulletin of Health Research. 2014;42(2):108–21.
- Centres for Diseases Control and Prevention. Vaccine Storage and Handling Tollkit. the U.S. Department of Health and Human Services (DHHS). the U.S. Department of Health and Human Services (DHHS); 2021. 493–493 p.
- Kemkes. Peraturan menteri kesehatan republik indonesia Nomor 12 tahun 2017 Tentang Penyelenggaraan imunisasi. Jakarta; 2017.
- WHO. Cold chain, vaccines and safe-injection equipment management. In: Department of Immunization VaB. Geneva: Department of Immunization, Vaccines and Biologicals; 2008.
- UNICEF. Handbook for Vaccine & Cold Chain. Department of Health & Family Welfare. India: Ministry of Health and Family Welfare; 2010.
- WHO. Temperature sensitivity of vaccines ed: Immunization. Vaccines and Biologicals; 2006.
- Chojnacky M, Miller W, Ripple D, Strouse G. Thermal Analysis of Refrigeration Systems Used for Vaccine Storage. America: Department of Commerce Gary Locke, Secretary; 2009.
- WHO. PATH. Domestic Refrigerators for Vaccine Storage in Tunisia Conclusion and Recommendations. USA: Vaccines and Biologicals; 2012.
- Anggita Y. Perancangan, Pembuatan, dan Pengujian Pengembangan Berpendingin Termoelektrik. Bandung: ITB; 2015.
- Dinkes. Profil Kesehatan Provinsi Jawa Barat Tahun 2013. Bandung: Dinas Kesehatan Kota Bandung; 2013.
- Kemenkes RI. Riset Kesehatan dasar Tahun 2013. Jakarta: Kementerian Kesehatan Republik Indonesia; 2013.
- Mishra A, Mishra S, Lahariya C, Jain P, Bhadoriya R, Shrivastav D. Practical observations from an epidemiological investigation of a measles outbreak in a district of India. Indian J Community Med. 2009;34(2):117–21.
- Thakur J, Ratho R, Bhatia S, Grover R, Issaivanan M, Ahmed B. Measles outbreak in a Periurban area of Chandigarh: need for improving vaccine coverage and strengthening surveillance. Indian J Pediatr. 2002;69(1):33–7.
- WHO. Annual Cold Chain Management Guide and Record. New Zealand: Ministry of Health; 2012.
- Wilsdon C. How Do Refrigerators Work? New York: ience in the Real World; 2010.
- Nurjanti S. Hubungan Tingkat Pengetahuan Bidan Tentang Imunisasi Dengan Perilaku Pengelolaan Vaksin Di Bidan Praktek Swasta Se-Wilayah Ranting Tengah Bantul Yogyakarta. Jurnal Kesehatan. 2010;1(1).
- Astuti M. Pengaruh Pengetahuan dan Kelengkapan Peralatan Rantai Dingin Terhadap Kualitas Pengelolaan vaksin Oleh Bidan Praktik Mandiri di Kota Bogor. Universitas Padjadjaran; 2013.
- Techathawat S, Varinsathien P, Rasdjarmrearnsook A, Tharmaphornpilas P. Exposure to heat and freezing in the vaccine cold chain in Thailand. Vaccine. 2007;25(7):1328–33.
- Yakum M, Ateudjieu J, Walter E, Watcho P. accine storage and cold chain monitoring in the North West region of Cameroon: a cross sectional study. BMC Res Notes. 2015;8(1):145.
- Kristini TD, Purwanti A, Udiyono. Faktor-Faktor Risiko Kualitas Pengelolaan Vaksin Yang Buruk Di Unit Pelayanan Swasta (Studi Kasus di Kota Semarang). Jurnal Kesehatan Masyarakat. 2008;3(2).
- WHO. Making use of Vaccine Vial Monitors. Geneva: Biologicals DoVa; 2000.
- Rahuh G, Suyitno H, Hadinegoro S, Kartasasmita C, Ismoedijanto, Soedjatmiko. Pedoman Imunisasi Di Indonesia. Jakarta: Badan Penerbit Ikatan Dokter Indonesia; 2014.
- Gomez PL, Robinson JM, Rogalewicz JA. Vaccine manufacturing. Vaccines. 2013;44.
- Mohan T, Verma P, Rao DN. Novel adjuvants & delivery vehicles for vaccines development: a road ahead. The Indian journal of medical research. 2013;138(5):779.
- World Health Organization. Temperature Sensitivity of Vaccines. CH-1211 Geneva 27, Switzerland: Department of Immunization, Vaccines and Biologicals; 2006.
- Chojnacky M, Rodriguez AL. Effect of thermal ballast loading on temperature stability of domestic refrigerators used for vaccine storage. PloS one. 2020;15(7):e0235777.

