Rebar Yahya Abdullah1*, Arazoo Issa Tahir2, Dlkhosh Shamal Ramadhan3, Zuhair Rushdi Mustafa4, Kawther Mohammed Galary5
1 MSc. (Maternity and Community Health Nursing Department, College of Nursing, University of Duhok,Kurdistan,Iraq).
2 MSc (Nursing Department, Bardarash Technical Institute, Duhok Polytechnic University, Kurdistan,Iraq).
3 MSc (Maternity and Community health nursing Department, College of Nursing, University of Duhok, Kurdistan, Iraq)
4PhD (Adult Nursing Department, College of Nursing, University of Duhok, Kurdistan, Iraq).
5 MSc (Maternity and Community Health Nursing Department, College of Nursing, University of Duhok, Kurdistan, Iraq).
*Corresponding Author: Rebar Yahya Abdullah, Maternity and Community Health Nursing Department, College of Nursing, University of Duhok, Kurdistan, Iraq.
E-mail: rebar.abdullah@uod.ac
Cita questo articolo
ABSTRACT
Background: Communities around the world have expressed concern about the safety and side effects of SARS-CoV-2 vaccines. The adverse effects of the Covid-19 vaccines played a critical role in public trust in the vaccines. The current study aimed to provide evidence on the side effects of the BNT163b2 mRNA COVID-19 vaccine (Pfizer-BioNTech®); ChAdOx1 nCoV-19 vaccine (AstraZeneca®); BBIBP-CorVvaccine (Sinopharm®) COVID-19 vaccines.
Material and Methods: A cross-sectional study design was performed from April 26th, 2021, to June 3rd, 2021. Convenience sampling was used to select respondents; face validity was performed to the mandatory multiple-choice items questionnaire to cover the respondent’s demographic characteristics, coronavirus-19 related anamneses, and the side effect duration of coronavirus-19 vaccines, the data were analyzed by using descriptive statistics.
Results: The 588 participants enrolled in the current study. ChAdOx1 nCoV-19 vaccine received 49.7%, followed by BNT163b2 mRNA COVID-19 vaccine and BBIBP-CorV (39.5% and 10.9%). The most common complaint was headache (61.2%), followed by vaccine injection site discomfort (58.8%), fatigue (49.7%), fever (48.3%), muscle discomfort (42.9%), and approximately (10.5% and 10.2%) had injection site swelling and nausea, respectively. Most of those surveyed had post-vaccine symptoms for one to two days (25.2%), (41%), and only a small percentage (3.7%) experienced them for over one month. ChAdOx1 nCoV-19 vaccine handled 53% of the side effects, followed by BNT163b2 mRNA COVID-19 vaccine (42%) and BBIBP-CorV vaccines (5%).
Conclusion: Prevalence of various local and systemic vaccines side effects, such as headache, fever, and pain at the injection site, was observed. Almost all participants had mild symptoms and were well-tolerated .AstraZeneca® vaccine has the most side effects, followed by the Pfizer® vaccine, and the Sinopharm® vaccine has the least. More independent studies on vaccination safety and public awareness are critical to improving public trust in vaccines.
Keywords: COVID-19; Vaccines; Side effects; Prevalence; Cross-sectional design.
INTRODUCTION
Millions of people around the world were infected by the Coronavirus Disease-2019 (COVID-19) within three months, until World Health Organization declared it as a pandemic on March 11, 2020 [1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a coronavirus that belongs to the Coronaviridae family’s Sarbecovirus subgenus, and a non-segmented positive-sense Ribonucleic acid (RNA) virus encompasses it [2]. Older individuals are at an increased risk of being infected with the SARS-CoV-2 [3]. Most vaccine options target the spike (S) protein. It is the principal target of neutralizing antibodies. It helps to neutralize antibodies to prevent the Angiotensin Converting Enzyme-2 (ACE2) receptor binding motif (RBM) from engaging with the host cell [4, 5]. The vaccine development for COVID-19 prevention has grown into a struggle between viruses and humans, which has made it more complicated, along with the discovery of other related strains. Many platforms are attempting to grow, with Deoxyribonucleic acid (DNA) and RNA-based platform showing the most promise [6]. Several countries have entered the vaccine development battle, hastening the clinical trial phase and attempting to produce an efficient and safe vaccine against COVID-19 [7]. The COVID-19 vaccines have been studied in large, randomized-controlled studies with people of all ages, genders, nationalities, and individuals with known medical disorders. Across all demographics, the vaccines have shown a high level of effectiveness and are safe and efficacious in patients with various underlying diseases [8]. According to a recent national study [9], the side effects of the COVID-19 vaccine were the most common reason for vaccine hesitancy among the population in the United Kingdom (U.K.). This finding was confirmed in the context of COVID-19 vaccinations, as fear of side effects has been cited as the primary reason for healthcare workers and students in Poland refusing to accept the Covid-19 vaccine [10, 11]. Vaccines are not completely free of side effects or complications [26], headache, nausea, pain, redness, and swelling are early adverse effects of vaccines that must be expected when taking vaccines [27]. Furthermore, conditions like blood clotting were suggested to be caused by the administration of COVID-19 vaccines from Pfizer, Moderna, and AstraZeneca. [28,29].The present study aimed to determine the prevalence of side effects of the COVID-19 vaccine among vaccinated people in the Kurdistan Region, Iraq.
MATERIALS AND METHODS
Study design
The study was conducted using a cross-sectional design from April 26th to June 3rd, 2021, in the Kurdistan region, Iraq.
Samples and sampling
An Internet-based study in the Kurdistan region of Iraq recruited to enroll a sample size of 588 people from people who had been vaccinated with one of the following vaccines: BBIBP-CorV, ChAdOx1 nCoV-19 vaccine, and BNT163b2 mRNA COVID-19 vaccines. However, illiterate and old age individuals were interviewed directly by authors to increase the sample representation. The individuals were invited by using invitation links in Viber™, Facebook™, and WhatsApp™ groups by using a non-random convenience sampling method. A Google™ form document was utilized to host and deliver the questions to responders. The inclusion criteria were participants who received one of the three mentioned COVID-19 vaccines and either received the first or second dose of the vaccine.
Instruments of the study
The self-administered questionnaire of the present study, composed of nine mandatory multiple-choice items, has been adapted from previous studies and World Health Organization data [12, 13]. The questionnaire was divided into four parts: the first part included demographic data, including gender, age, and profession; the second part dealt with COVID-19 history, including COVID-19 previous infection, type and dose of COVID-9 vaccines, and medical history like having any chronic disease; the third part included the side effects and side effect duration of COVID-19 vaccines.
Statistical analysis
The descriptive statistics were performed to determine the study variables; age, gender, occupation, and the data that related to the COVID-19 vaccine. The current study used SPSS version 23 for the descriptive statistics.
RESULTS
588 participants in the study. Nearly two-thirds of participants were males (64.3%); their mean age was 41.5 years and ranged between 18 and 65 years. Most of the participants were healthcare workers (31.3%), government employees (25.2%), jobless (19), students (18), and self-employed (6.5%), as shown in Table 1.
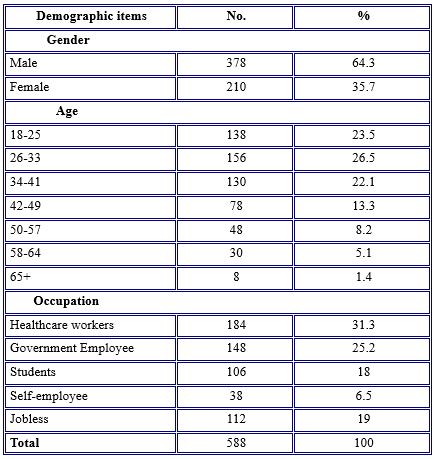
Table 1. Demographic Characteristics of study participants
According to some questions stated in Table 2, nearly half (46.3%) of the participants did not infect before taking the vaccine. About (40.8%) reported that they were infected with COVID-19 previously. Compared with a tiny percentage (12.9%) having the vaccine without knowing whether they were infected with the COVID-19 virus or not.
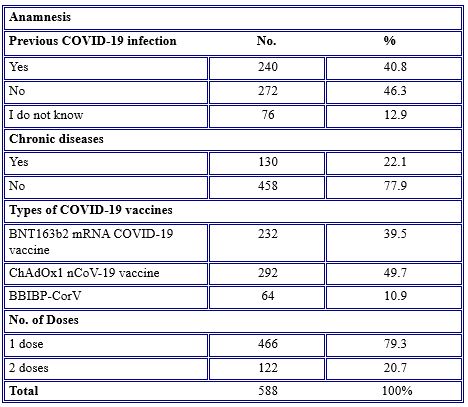
Table 2. COVID-19 vaccines related anamnesis
Regarding chronic diseases among the participants who had the COVID-19 vaccine, over three-quarters (77.9%) had no chronic diseases. The most common types of vaccines received by the participants were ChAdOx1 nCoV-19 vaccine (49.7%), followed by BNT163b2 mRNA COVID-19 vaccine and BBIBP-CorV (39.5% and 10.9%). Regarding the number of vaccine doses gained, over three-quarters (79.3%) of participants had a single dose of vaccine at the time of the study.
Regarding the response of the participants toward COVID-19 side effects, they reported having at least one side effect after the COVID-19 vaccine job. The most common side effects among the study population (61.2%) were headaches, followed by vaccine injection site pain (58.8%), fatigue (49.7%), fever (48.3%), muscle pain (42.9%), and nearly the same percentage (10.5% and 10.2%) complained of injection site swelling and nausea, respectively. Rarely (0.3% and 0.7%) reported mouth ulcers and tonsillitis, side effects of the vaccine, as noted in Table 3.
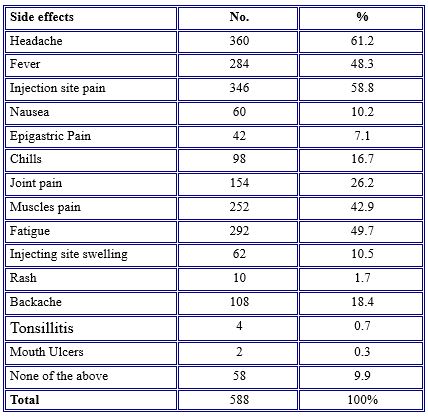
Table 3. Prevalence of COVID-19 vaccine side effects among study participants
Table 4 shows that, for the duration of the occurrence of side effects, the vast majority (41.5%) of the participants had post-vaccination side effects for about two days, while 25.2% had them for one day, and 10.9% of the individuals complained about side effects for three days. 3.7% of them had a longer duration of side effects for over one month.
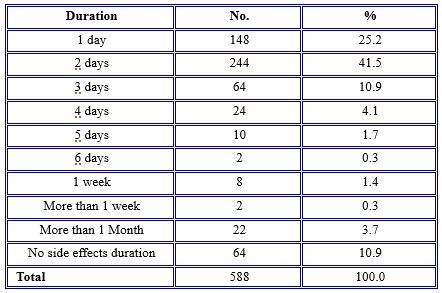
Table 4. The duration of side effects of COVID-19 vaccines
Regarding side effect prevalence with different age groups, symptoms were more common among the younger age groups ranging from 18 to 57 years old. Symptoms were much less severe in older age groups (58–64), with no noticeable side effects observed in participants older than 60 years old. Headache was more common in the age group 34-41 years old (14.2%); injection site pain was more common in the age group 26-33 years old (16.6%); fatigue was more common in the age group 34-41 years old (13.2%) as in Table 5.
Concerning the occurrence of side effects among BNT163b2 mRNA COVID-19 vaccine, ChAdOx1 nCoV-19 vaccine, and BBIBP-CorV vaccines, the vast majority (53%) of the side effects were because of ChAdOx1 nCoV-19 vaccine, followed by BNT163b2 mRNA COVID-19 vaccine (42%), BBIBP-CorV vaccines (5%) were safer than BNT163b2 mRNA COVID-19 vaccine and ChAdOx1 nCoV-19 vaccine vaccines in that almost all side effects occurred among vaccinated individuals.
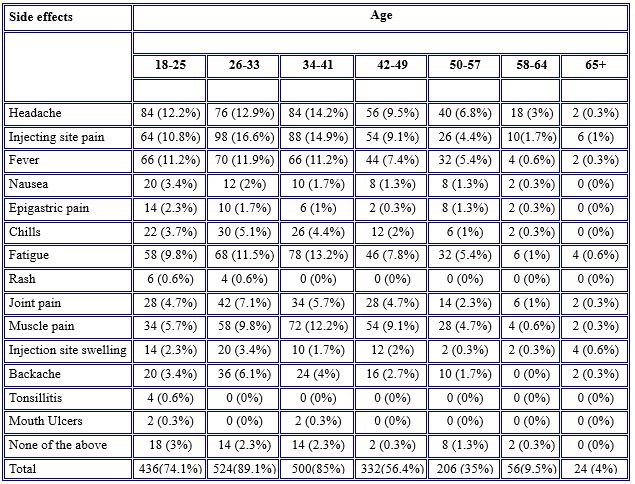
Table 5. Prevalence of the side effects of COVID-19 vaccines among age groups
Some side effects such as nausea, epigastric pain, chills, injection site swelling, backaches, tonsillitis, and mouth ulcers have not occurred at all. Only a few participants (43.7%, 28.1%, 21.9%, and 18.7%, respectively) experienced injection site pain, fatigue, headache, and fever after receiving the BBIBP-CorV vaccine.
Most of the symptoms were observable in those who received the ChAdOx1 nCoV-19 vaccine and BNT163b2 mRNA COVID-19 vaccine vaccines, although symptoms were more common in individuals vaccinated with ChAdOx1 nCoV-19 vaccine. Common side effects between BNT163b2 mRNA COVID-19 vaccine and ChAdOx1 nCoV-19 vaccine were headache (69 % versus 63.7 %), injection site pain (56.8 % versus 63.6 %), fever (42.2 % versus 59.5 %), fatigue (56.8 % versus 48.6%), and muscle pain (44.8 versus 47.9 %) as shown in Table 6.
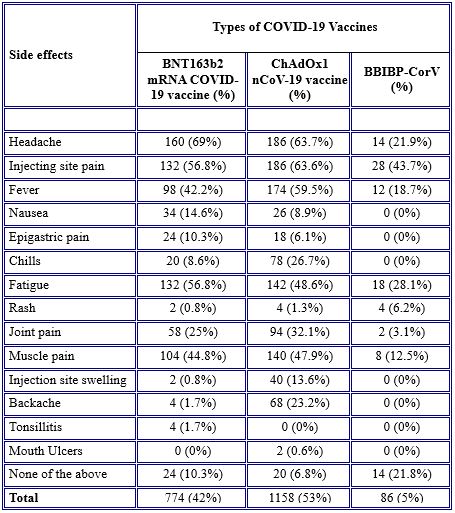
Table 6. Occurrence of side effects between vaccines
DISCUSSION
During the pandemic of COVID-19, the World Health Organization recommended that all nations strive to maintain population immunization. Although legislation and policies in this region are different, they still emphasize people at risk of coronavirus disease, such as healthcare workers, the elderly, and patients with chronic conditions [14]. Thus, the results of the current study showed that most of the participants (31.3%) were healthcare workers (males 64.3%), and most of them (46.3%) did not affect COVID-19. A similar study was conducted in India, which stated that, according to government regulations, the vaccine was initially administered to healthcare personnel in both government and private hospitals throughout India [15]. Correspondingly, in the US, priority is given mainly to all healthcare workers, then individuals who have an underlying condition, and after that to all essential service workers and older adults [16].
Because of the speed of COVID-19 vaccine manufacturing, concerns among the public have emerged about the safety of these new vaccines. No serious safety problems were reported [17]. Overall, COVID-19 vaccines are safe and will protect the community from developing severe COVID-19 disease and dying from COVID-19. BNT163b2 mRNA COVID-19 vaccine is an mRNA-based vaccine, ChAdOx1 nCoV-19 vaccine is an Adenovirus vaccine, and BBIBP-CorV is a vaccine [18]. According to the research, COVID-19 vaccination adverse effects are characterized as either local or systemic reactions, with severity ranging from mild to moderate [19]. The mRNA-based vaccines such as BNT163b2 mRNA COVID-19 vaccine have the highest level of side effects reported, except for diarrhea and arthralgia [20]. Since some of the vaccinated individuals in the current study received the mRNA-based vaccines, they were not free from side effects. No serious events associated with the COVID-19 vaccines, such as vaccine-induced immune thrombotic thrombocytopenia reported. However, most of the side effects were common and non-life-threatening. The side effects were systematic and local. The systemic reactions were headache (61.2%), fatigue (49.7%), fever (48.3%), muscle pain (42.9%), joint pain (26.2%), backache (18.4%), chills (16.7%), nausea (10.2%), epigastric pain (7.1%), and rash (1.7%), whereas the local reactions were injection site pain and injection site swelling (50.8%) and (10.5%), respectively. The rarest side effects were tonsillitis (0.7%) and mouth ulcers (0.3%). These findings are in line with those reported in the literature and reported by the Food and Drug Administration (FDA), which are: injection site pain, fatigue, headache, fever, chills, muscle pain, and joint pain are common side effects of COVID-19 vaccines [21, 15]. Similar findings were observed in the Czech Republic where the most common side effects among vaccinated individuals were injection site pain, fatigue, headache, muscle pain, and feeling unwell [12]. Also, a retrospective cross-sectional study was conducted among Saudi residents to study the side effects of the BNT163b2 mRNA COVID-19 vaccine. The study found that the most common symptoms were injection site pain, fever, headaches, flu-like symptoms, and tiredness. Less common side effects were tachycardia, generalized body aches, shortness of breath, joint pain, chills, and drowsiness. Rare side effects were tenderness, lymph node swelling, and Bell’s palsy [22]. In contrast to our study, in a systematic review study, the most common side effects were arthralgia (20). Mild to moderate side effects are experienced by vaccinated individuals. They are signs that the immune system of the body is responding to the vaccine and building protection against the COVID-19 virus (23/24). Also, in the present study, we found that the duration of post-vaccination side effects varied among participants. The majority (41.5%) were complaining about the side effects for two days, whereas 25.2% had side effects for one day, and 10% for three days. Only 3.7% had long-duration side effects for over one month. These findings follow the current studies which state that most of the side effects occur within the next 3 days after vaccination [15]. Also, similar findings were reported by Riad et al., [12]. They found that the duration of general side effects following the vaccine was mainly one day (45.1%) or three days (35.8%), and only 1.4% of them had lasted over a month.
Also, it is important to highlight that the prevalence of side effects was higher among younger individuals (> 49 years old) and almost no noticeable side effects occurred among older participants (60 years old). These findings are consistent with those published by the FDA, which found that injection site pain, weariness, headache, and muscle soreness were more common in the 55-year-old group than in the > 55-year-old group [21, 15]. Also, the same findings reported among the Czech Republic and Saudi residents, respectively [12], reported that younger adults 43 years old were more frequently affected by side effects, and [22] concluded that the frequency of side effects was higher in individuals younger than 60 years of age, except for injection site pain, which was more frequent among those 60 years old.
Concerning the comparison of the occurrence of side effects between BNT163b2 mRNA COVID-19 vaccine, ChAdOx1 nCoV-19 vaccine, and BBIBP-CorV vaccines, the findings of the present study revealed that there were substantial variations between these vaccines in the presence of side effects. The majority (53%) of side effects were because of ChAdOx1 nCoV-19 vaccine, followed by BNT163b2 mRNA COVID-19 vaccine (42%) except for headache, nausea, epigastric pain, fatigue, and tonsillitis which were more sever in BNT163b2 mRNA COVID-19 vaccine than ChAdOx1 nCoV-19 vaccine. The current study found BBIBP-CorV vaccine was safer than BNT163b2 mRNA COVID-19 vaccine and ChAdOx1 nCoV-19 vaccine vaccines in all side effects that occurred among vaccinated individuals. This finding is supported by a systematic review and meta-analysis of randomized control trials (RCTs), which revealed that those who received mRNA-based vaccines had higher rates of side effects in reactogenicity [20]. The same findings were documented in Jordan. 2213 individuals received BBIBP-CorV, ChAdOx1 nCoV-19 vaccine, BNT163b2 mRNA COVID-19 vaccine, and other vaccines. They found that those who received the ChAdOx1 nCoV-19 vaccine reported the most abundant post-vaccination symptoms, while most of those who received the BBIBP-CorV vaccine were free from symptoms [23]. Another study was conducted to assess the symptoms following the COVID-19 vaccine among residents in India. 5396 people responded to the survey. The findings revealed that the frequency of experiencing symptoms following the BBIBP-CorV vaccine was less (24.4%) compared to BNT163b2 mRNA COVID-19 vaccine 70.7% [25]. As seen, the BBIBP-CorV vaccine has few side effects compared to other vaccines.
CONCLUSIONS
The most common side effect of the BNT163b2 mRNA COVID-19 vaccine, ChAdOx1 nCoV-19 vaccine, and BBIBP-CorV among the vaccinated population of the current study was headaches, injection site pain, injecting site swelling, fatigue, fever, muscle pain, joint pain, backache, chills, nausea, epigastric pain, and rash. These side effects were consistent with the data reported in the literature. Most of these side effects were mild, and no serious incidents were documented. Symptoms were more common in younger people. Although data reported in the literature showed that mRNA-based vaccines such as BNT163b2 mRNA COVID-19 vaccine had higher side effects, However, the current study found that the ChAdOx1 nCoV-19 vaccine, which is an adenovirus-based vaccine, had more side effects than other vaccines, and the BBIBP-CorV vaccine had the lowest side effects compared to the ChAdOx1 nCoV-19 vaccine and BNT163b2 mRNA COVID-19 vaccine vaccines.
Limitations
The limitation of the current study is that it was difficult to measure the severity of the side effects because the study is a survey-based technique. Thus some side effects needed to be measured by using instruments or tools, for instance, measuring body temperature by the thermometer to know the severity of fever, and using a pain scale to measure headache, joint pain, and muscle pain. Also it is prone to selective bias as it is internet based study, not everyone has equal chance to be included in the study. Further studies needs to be done with more representative samples concerning COVID-19 intention.
Conflict of interest
The authors have no conflict of interest to declare.
Funding
The current study was not funded by any financial resources.
Ethics
The present study was carried out in accordance with the Helsinki Declaration”. Study approval was obtained by written authorization of the Ethics Committee of the College of Nursing at Duhok University. The approval is without a serial number and verbal informed consent has been obtained from each participant before participation in the current study.
Acknowledgments
We would like to extend our appreciation to all respondents who took part in this online survey. We highly appreciated their time and effort.
REFERENCES
- World Health Organization. Novel Coronavirus (2019-nCoV) SITUATION REPORT – https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf. .
- Zhuo Zhou , Lili Ren , Li Zhang , Jiaxin Zhong , Yan Xiao , Zhilong Jia et alHeightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe .2020; 27, 883-890.e2.
- Centers for Disease Control and Prevention. COVID-19 information page (https://www.cdc.gov/coronavirus/2019 -ncov/index.html).
- Addetia A, Crawford KHD, Dingens A, Zhu H, Roychoudhury P, Huang ML, Jerome KR, Bloom JD, Greninger AL. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J Clin Microbiol. 2020 Oct 21;58(11):e02107-20. doi: 10.1128/JCM.02107-20. PMID: 32826322; PMCID: PMC7587101.
- Thompson, C. P., Grayson, N. E., Paton, R. S., Bolton, J. S., Lourenço, J., Penman, B. S., Lee, L. N., Odon, V., Mongkolsapaya, J., Chinnakannan, S., Dejnirattisai, W., Edmans, M., Fyfe, A., Imlach, C., Kooblall, K., Lim, N., Liu, C., López-Camacho, C., McInally, C., … Girvan, M.. Detection of neutralising antibodies to SARS-CoV-2 to determine population exposure in Scottish blood donors between March and May 2020. Eurosurveillance, 25(42). https://doi.org/10.2807/1560-7917.ES.2020.25.42.2000685
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. New England Journal of Medicine..2020; 21;382(21):1969-73.
- Caddy S. Developing a vaccine for covid-19. BMJ 2020;369:m1790 doi: 10.1136/bmj.m1790
- World Health Organization. . Safety of covid-19 vaccines. https://www.who.int/news-room/feature-stories/detail/safety-of-covid-19-vaccines.2020.
- Luyten, J.; Bruyneel, L.; van Hoek, A.J. Assessing vaccine hesitancy in the UK population using a generalized vaccine hesitancy survey instrument. 2019;37, 2494–2501. [CrossRef] [PubMed] .
- Szmyd, B.; Bartoszek, A.; Karuga, F.F.; Staniecka, K.; Błaszczyk, M.; Radek, M. Medical Students and SARS-CoV-2 Vaccination: Attitude and Behaviors. 2021; 9, 128. [CrossRef].
- Szmyd, B.; Karuga, F.F.; Bartoszek, A.; Staniecka, K.; Siwecka, N.; Bartoszek, A.; Błaszczyk, M.; Radek, M. Attitude and Behaviors towards SARS-CoV-2 Vaccination among Healthcare Workers: A Cross-Sectional Study from Poland. Vaccines 2021, 9, 218. https://doi.org/10.3390/vaccines9030218
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Košˇcík, M.; Klugar, M.Prevalence of COVID-19 Vaccine Side Effects among HealthcareWorkers in the Czech Republic. Clin. Med., 10, 1428. https://doi.org/ .2021;10.3390/jcm10071428.
- World Health Organization. . Side Effects of COVID-19 Vaccines. https://www.who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines.2021
- World Health Organization. Guidance on routine immunization services during COVID-19 pandemic in the WHO European region: World Health Organization. Regional Office for Europe.2020.
- Das, L., Meghana, A., Paul, P., & Ghosh, S. Are We Ready For Covid–19 Vaccines?–A General Side Effects Overview. Journal of Current Medical Research and Opinion.2021; 4(02).
- Barnabas, R. V., & Wald, A. A public health COVID-19 vaccination strategy to maximize the health gains for every single vaccine dose: American College of Physicians.2021.
- Gee, J. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. Morbidity and mortality weekly report. 2021; 70.
- Wise, J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots: British Medical Journal Publishing Group.2021.
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine-United States, December 2020. Morb. Mortal. Wkly. Rep.2021, 5152, 1653–1656. [Google Scholar] [CrossRef]
- Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, Turner RJ. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. 2021; 9(5):467. https://doi.org/10.3390/vaccines9050467.
- Centres for Diseases Control and Prevention. Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Pfizer-BioNTech COVID-19 Vaccine. Retrieved 5 June, from https://cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.2021.
- El-Shitany, N. A., Harakeh, S., Badr-Eldin, S. M., Bagher, A. M., Eid, B., Almukadi, H., Sindi, N. Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine Among Saudi Residents: A Retrospective Cross-Sectional Study. International journal of general medicine.2021;14, 1389.
- Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM, Mohamud R. Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects. 2021; 9(6):556. https://doi.org/10.3390/vaccines9060556
- World Health Organization. Side Effects of COVID-19 Vaccines. Retrieved 4 June, 2021, from https://who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines.2021
- Jayadevan, R., Shenoy, R. S., & Anithadevi, T. Survey of symptoms following COVID-19 vaccination in India. medRxiv.2021.
- Kimmel SR. Vaccine adverse events: separating myth from reality. Am Fam Physician. 2002;66(11):2113–20.
- Center for Disease Control and Prevention. Reactions and adverse events of the pfizer-BioNTech COVID-19 vaccine | CDC [Internet]. Centers Dis Control Prev. 2020 [accessed 2021 May 30]. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfi zer/reactogenicity.html
- Lee E, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, Semple JW, Arnold DM, Godeau B, Lambert MP, et al. Thrombocytopenia following Pfizer and moderna SARS-CoV-2 vaccination. Am J Hematol. [Internet]. 2021 [accessed. 2021 Aug 31];96:534–37. /pmc/articles/PMC8014568/ .
- European Medicines Agency. AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets [Internet]; 2020 [accessed 2021 May 30].
The questionnaire
(COVID-19 Vaccines Side Effects Among Iraqi people In Kurdistan Region)
Dears the aim of this survey is to determine the (Prevalence of Covid-19 Vaccines side effects). We are grateful for filling in this survey from you and your family who got vaccinated. I would like to assure you that your answers will remain confidential and your personal details are not required. Also, your answers will be on online systems only.
- Age
18_25
26_33
34_41
42_49
50_57
57_64
65 and more
- Gender
Male
Female
- Occupation
Health care workers
Employee Government
Student
Own Job
Jobless
- Do you have any chronic disease?
Yes
No
- Did you infected with Covid-19 before?
Yes
No
I don’t know
- Which Covid-19 Vaccine you took it?
Sinopharm
Astrazenea
Pfizer
- How many Doses you got it?
1 dose
2 doses
- Select the vaccine side effects that occurred with you
Headache
Vaccine injection site pain
Fever
Nausea
Epigastric pain
Chills
Joint pain
Muscles pain
Fatigue
Injection site swelling
Allergy
Backache
Tonalities
Mouth Ulcers
No one
- Duration of side effects of Covid-19 Vaccines
1 day
2 days
3 days
4 days
5 days
6 days
1 week
More than 1 week
More than 1 month
No duration
![]() This work is licensed under a Creative Commons
This work is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License.

