Vidal Navarro Ana 1. Férriz Tena Náyades 1. Carreres Giménez María Encarnación 1. Verdu Quirant Trinidad 1. Menchón Simón María de las Nieves 2. Campello García María José 2. Serrano López Juan Francisco 2 and Soler Climent Esther 3*.
- Primary Care. Health Department Elche General Hospital, Elche, Alicante, Spain; FISABIO, Valencia, Spain.
- Clinical analysis laboratory. Health Department Elche General Hospital, Elche, Alicante, Spain; FISABIO, Valencia, Spain.
- Research and Innovation Area. Health Department Elche General Hospital, Elche, Alicante, Spain; FISABIO, Valencia, Spain.
* Corresponding author: Soler Climent, Esther. E-mail: soler_estcli@gva.es
Vidal Navarro Ana, Férriz Tena Náyades, Carreres Giménez María Encarnación, Verdu Quirant Trinidad, Menchón Simón María de las Nieves, Campello García María José, Serrano López Juan Francisco, Soler Climent Esther
Review article
DOI:10.32549/OPI-NSC-106
Submitted: 16 April 2024
Revised: 19 june 2024
Accepted: 25 june 2024
Published online: 26 june 2024
License: This article is licensed under the Creative Commons Attribution - Non Commercial - No Derivatives 4.0 (CC BY NC ND 4.0) international license.
Abstract:
This study investigates the impact of smart refrigerators on the logistics and management of biological samples, emphasizing the critical phases of transport and storage to enhance the pre-analytical quality of blood samples. Efficient sample management is crucial for ensuring diagnostic accuracy.
Cite this article
ABSTRACT
Introduction: This study investigates the impact of smart refrigerators on the logistics and management of biological samples, emphasizing the critical phases of transport and storage to enhance the pre-analytical quality of blood samples. Efficient sample management is crucial for ensuring diagnostic accuracy.
Objective: The primary objective is to evaluate the efficiency of smart refrigerators equipped with cloud technology in optimizing the transport and storage of biological samples. The secondary objective is to assess healthcare personnel’s perceptions and satisfaction with these technologies.
Method: A longitudinal prospective analysis was conducted to assess both the quantitative incidence of pre-analytical errors and the qualitative perceptions of healthcare personnel regarding these technologies. Samples were collected from six primary care centers within the Elche General Health Department, with one center using smart refrigerators and five using conventional methods. The refrigerators featured GPS, real-time temperature sensors, alert systems for cold chain interruptions, and RFID technology. Training on refrigerator use, systematic data collection on pre-analytical errors, and surveys and semi-structured interviews with healthcare personnel were conducted. Descriptive methods and hypothesis testing, including Z-statistics and logistic regression models, were used for statistical analysis.
Results: The analysis revealed a significant decrease in the incidence of coagulated and hemolyzed samples at the center using smart refrigerators. Specifically, the rate of coagulated samples was reduced by 69.39%, while hemolyzed samples decreased by 78.12%. This improvement contrasts with trends observed in centers using conventional practices. A significant 94.62% of the staff reported a positive experience with the smart refrigerators, highlighting high satisfaction and the importance of stricter control in handling and transporting samples to prevent errors.
Conclusions: The use of smart refrigerators in the transport and storage of biological samples effectively improves the pre-analytical quality of blood samples, reduces pre-analytical errors, and enhances staff satisfaction. These findings underscore the importance of incorporating advanced technologies in the management of biological samples in primary care settings.
Keywords: Pre-Analytical Phase, Smart Refrigerators, Pre-analytical Error, Medical Laboratory, Blood Preservation.
INTRODUCTION
In recent decades, the imperative to reduce costs has driven the healthcare sector towards the consolidation and centralization of diagnostic services in high-capacity laboratories [1]. This transformation underscores the critical importance of efficiently transporting various biological samples from peripheral collection points to centralized analysis centers, highlighting the need for optimized logistics and meticulous sample management to ensure diagnostic accuracy [2]. As emphasized by Giavarina and Lippi, precision is crucial at every stage of the diagnostic process [3]. The ability of clinical laboratories to deliver reliable results depends on the rigorous implementation of standardized procedures throughout the pre-analytical, analytical, and post-analytical phases of the diagnostic process. Studies by Rak-Pasikowska et al. and Carraro et al. highlight frequent errors and the nature of these in the pre-analytical phases, illustrating common challenges and underscoring the importance of addressing them to enhance overall diagnostic quality [4,5]. The pre-analytical phase is particularly complex, involving numerous variables that can significantly impact the quality of analytical results. These variables range from the patient’s physiological conditions to the methodologies used in sample collection, underscoring the necessity for meticulous and standardized management at this crucial stage [6,7]. It is important to recognize that the pre-analytical phase comprises two sub-stages: extralaboratory and intralaboratory, each with its own set of challenges and potential sources of error, which can be difficult to quantify due to the variability in their occurrence [8,9]. Efforts to manage the pre-analytical phase more effectively have been documented extensively. Akande discusses the quality management of the pre-analytical phase, emphasizing monitoring and control to mitigate errors before they affect the test outcomes [10]. Similarly, Lee’s work on reducing pre-analytical errors through quality improvement activities in a university hospital setting showcases practical applications of these principles [11]. Cornes reviews the evolution of the pre-analytical phase and projects future advancements that could further reduce errors [12]. Rodríguez-Ravelo and Marcel highlight the broad impact of pre-analytical variables on clinical laboratory results [13]. Lastly, innovations such as the specialized Vacutainer systems play a crucial role in improving sample integrity during collection and transport [14]. Moreover, Lippi et al. highlight that the quality of blood samples, particularly the prevalence of hemolyzed samples, is a frequent cause of specimen nonconformity in clinical laboratories, indicating the urgent need for strategies to improve the quality of blood samples [15]. Tóth et al. demonstrated that hemolysis is a common pre-analytical problem in both newborns and adults, significantly affecting analysis results [9,16]. Bostic et al. reported that their laboratory managed to reduce the number of insufficient samples for coagulation tests by tracking and eliminating expired blood collection tubes, suggesting an effective strategy to minimize this type of pre-analytical error [17]. In another study, Lippi et al. showed how brief venous stasis during extraction can significantly alter the results of coagulation tests, emphasizing the importance of standardizing the venipuncture process [18]. Van Geest-Daalderop et al. evaluated the effect of pre-analytical variables, including the time between blood collection and PT/INR determinations, and found significant effects based on storage conditions and handling, recommending a maximum of 6 hours between blood collection and PT/INR determination [19].
These findings underline the interrelationship between pre-analytical management, diagnostic accuracy, and operational efficiency, stressing the continuous need for quality improvement throughout all phases of the diagnostic process, especially in standardizing pre-analytical procedures. The prevalence of pre-analytical errors varies significantly between different countries, reflecting global variability in sample handling practices and standards [8,13]. Hemolysis, in particular, frequently undermines the accuracy of critical tests such as INR and aPTT [20,21]. Economically, pre-analytical errors, especially those resulting from hemolyzed samples, impose a substantial financial burden on health institutions, significantly affecting hospital budgets [10,22]. Detailed analysis of hemolysis causes reveals a range of factors, from collection techniques to sample transport, highlighting the need for careful and standardized practices to minimize its incidence [23,24].
Proper management of blood sample transport is essential for precise and reliable clinical analyses. Recent studies emphasize how mechanical agitation during the transport of non-centrifuged samples can significantly affect test results, underscoring the importance of selecting transportation methods that preserve sample integrity [25]. The implementation of advanced technologies, such as smart refrigerators, offers a promising solution. These devices integrate cutting-edge temperature and vibration controls essential for maintaining optimal conditions during transport [26]. Their ability to ensure a stable environment and monitor temperature in real-time is a crucial advancement in preventing sample coagulation or degradation. The significance of these findings necessitates specific research to explore the impact of smart refrigerators on the pre-analytical process. Despite the lack of direct evidence evaluating these technologies in specific contexts, it is imperative to investigate their potential to enhance the traceability and control of blood samples, particularly in primary care. This approach could not only mitigate pre-analytical errors but also enhance the quality and reliability of clinical analyses.
OBJECTIVE
Main objective
To evaluate the efficiency and applicability of smart refrigerators integrated with cloud-based systems for the transport of biological samples.
This study primarily investigates the potential of these advanced refrigeration and monitoring systems to improve control over the cold chain and reduce the potential for vibration and impacts from collection to final destination. However, it is acknowledged that due to the lack of comprehensive data and investigation, conclusions regarding their effect on pre-analytical errors are preliminary and largely based on the surrogate endpoint of hemolysis reduction.
Secondary objectives
- To determine health personnel’s perception and evaluation of the impact that repeated sample collection has on their routine clinical work.
- To examine satisfaction with the use of smart refrigerators at the end of the study period through interviews with all health professionals involved in the sample handling, transfer, and processing stages.
MATERIAL AND METHODS
Study Design
This study employs a prospective longitudinal design to evaluate the efficiency of smart refrigerators in transporting biological samples from primary care centers to a central laboratory. It utilizes both quantitative and qualitative methods to assess not only the incidence of pre-analytical errors but also the healthcare staff’s perception of the need for action and their satisfaction with these technologies.
Population and Sample
The study analyses blood samples collected from six primary care centers within the Elche General Health Department, serving 168,975 people. It compares pre-analytical problems at one center using smart refrigerators to send samples to the central laboratory with five centers that did not use them. Questionnaires were administered to 97 health professionals to assess the impact of repeated sample collection. Additionally, interviews were conducted with all personnel involved in sample handling, transport, and processing to assess satisfaction with the refrigerators.
Materials
Smart refrigerators equipped with GPS tracking, real-time temperature sensors (maintaining 2°C to 8°C), alerts for cold chain interruptions, RFID (Radio Frequency Identification) access control, and anti-vibration systems were used. The use of RFID access control ensures a secure and efficient system for tracking and managing access to the refrigerators, preventing unauthorized entry and maintaining the integrity of the samples by ensuring that only authorized personnel can handle them. This technology enhances traceability and accountability, as every access event is recorded, providing a detailed audit trail that helps in monitoring the handling process and improving overall security and reliability.
Control of Confounding Variables
To ensure internal validity, differences in staff training, transport conditions, and sample collection procedures were meticulously controlled through standardized protocols across all centers. Staff experience, the professional-to-population ratio, and professional rotation were consistent among the centers.
Procedures
The study involved training personnel in the use of smart refrigerators, systematic data collection on pre-analytical incidents, and conducting surveys and semi-structured interviews with health personnel.
Instrument Validation
Questionnaires and interviews underwent a validation process, including expert review and a pilot test, followed by adjustments based on factor analysis and item reliability (Cronbach’s Alpha).
Statistical Analysis
Data are presented as number and percentage for categorical variables, and continuous data are expressed as mean ± standard deviation (SD).
Advanced statistical techniques were applied, starting with descriptive analyses to understand the basic characteristics of the data. Hypothesis-testing methods, including the Z statistic for comparing proportions, Odds Ratios (OR) with 95% CI, and logistic regression models, were used to explore the relationship between the use of smart refrigerators and the reduction of pre-analytical errors.
To assess the reliability of the questionnaires administered to healthcare professionals, Cronbach’s alpha value was calculated to determine the internal consistency of measurement instruments.
Finally, all tests with p-value< 0.05 were considered significant. Data were analyzed using R software.
Ethics and Research Integrity
All procedures were reviewed and approved by the institutional ethics committee. Informed consent was obtained from all participants, ensuring confidentiality and ethical data handling.
Ethics committee protocol number: PI 89_2022_NIPAP-22. The project received approval on September 6, 2022.
RESULTS
The initial questionnaire, which evaluated perceptions of pre-analytical issues and their frequency, achieved a Cronbach’s alpha value of 0.87, indicating high reliability. Similarly, the final questionnaire aimed at assessing satisfaction with the use of smart refrigerators showed a Cronbach’s alpha value of 0.91, reflecting excellent internal consistency. These results suggest that both questionnaires were reliable and consistent instruments, validating the methodological robustness of the study and reinforcing confidence in the findings obtained.
The data in Table 1 provide a comprehensive view of healthcare personnel’s perceptions regarding the challenges and critical areas in the pre-analytical phase of blood sample analysis.
| Question | Responses |
| How often is repeating the blood analyses of the patients necessary? | Hardly ever: 30.93% (n=30), Sometimes: 54.64% (n=53), Often: 8.25% (n=8), Very often: 3.09% (n=3), Do not know/do not answer: 3.09% (n=3) |
| In your opinion, where do you think lies the biggest source of errors in blood analyses? | Specimen transport: 24.74% (n=24), Specimen preparation and analysis: 14.43% (n=14), Specimen storage conditions: 19.59% (n=19), Specimen collection: 23.71% (n=23), Protocol non-compliance by the patient: 11.34% (n=11), Do not know/do not answer: 6.19% (n=6) |
| Which measures do you think should be taken in order to avoid errors in blood analyses? | Considering the patients: 16.49% (n=16), Considering the staff: 20.62% (n=20), Considering the training: 23.71% (n=23), Considering monetary resources: 3.09% (n=3), Considering the available material resources: 29.90% (n=29), Do not know/do not answer: 6.19% (n=6) |
| How would you rate the current storage and transport of specimens system of the primary care centre? | Bad: 3.09% (n=3), Fair: 16.49% (n=16), Good: 55.67% (n=54), Excellent: 8.25% (n=8), Do not know/do not answer: 16.49% (n=16) |
| Do you think a higher control of the influential variables in transport and storage of the specimens would have a positive influence for avoiding errors? | Yes: 84.54% (n=82), No: 8.25% (n=8), Do not know/do not answer: 7.22% (n=7) |
Table 1. Percentage of answers in relation to experiences and perception of the healthcare staff.
According to Table 1, a significant majority of respondents (54.64%) reported that it is “sometimes” necessary to repeat blood tests due to errors, highlighting the frequency of pre-analytical issues encountered in their daily routine.
When asked about the primary source of errors in blood tests, respondents identified several key areas: sample transportation (24.74%, n=24), sample collection (23.71%, n=23), and storage conditions (19.59%, n=19). These areas were highlighted as the most critical points where errors occur, emphasizing the need for specific interventions. Regarding measures to prevent errors in blood tests, the responses varied. The most frequently suggested measures included considering the available material resources (29.90%, n=29) and focusing on staff training (23.71%, n=23). Other important areas included addressing patient-related factors (16.49%, n=16) and considering healthcare personnel (20.62%, n=20).
When rating the current storage and transportation systems in their primary care centers, most respondents rated them as “Good” (55.67%, n=54) while 16.49% (n=16) rated them as “Fair” and 8.25% (n=8) as “Excellent.” However, a notable 16.49% (n=16) were unsure or did not respond. Finally, when asked if increased control over variables affecting the transportation and storage of samples would positively impact error reduction, a large majority (84.54%, n=82) believed it would. This consensus underscores the importance of strict controls in these areas to improve the accuracy and reliability of blood tests.
| Question | Domain
Likert scale |
Mean ± SD |
| How would you rate the impact that, in your view, the repetition of specimens collection has on the patients’ lives? | Impact
1 to 10 (1 = no impact; 10 = highest impact) |
7.05 ± 0.65 |
| How would you rate the impact that, in your view, the repetition of specimens collection has on the primary care centre? | 7.52 ± 0.41 | |
| How would you rate the efficiency of work performed in the pre-analytical phase of the specimens in this primary care centre? | Efficiency
1 to 10 (1 = poor; 10 = excellent) |
7.69 ± 0.61 |
| Do you think that the Collection Phase needs more urgent attention in order to improve the efficiency in the pre-analytical phase of the specimens in this primary care centre? | Priority
1 to 5 (1 = lowest; 5 = highest) |
3.82 ± 0.41 |
| Do you think that the Storage Phase needs more urgent attention in order to improve the efficiency in the pre-analytical phase of the specimens in this primary care centre? | 4.20 ± 0.41 | |
| Do you think that the Transport Phase needs more urgent attention in order to improve the efficiency in the pre-analytical phase of the specimens in this primary care centre? | 4.25 ± 0.55 | |
| Do you think that the Handling Phase needs more urgent attention in order to improve the efficiency in the pre-analytical phase of the specimens in this primary care centre? | 4.48 ± 0.5 |
Table 2. Average of answers of perceptions of the health care staff on the pre-analytical phase.
The perceived need for repetition significantly impacts both patients’ lives and the functioning of the health center, with mean scores of 7.05 ± 0.65 and 7.52 ± 0.41 respectively. The assessments also highlight handling and transportation as critical pre-analytical sub-stages requiring immediate attention, with urgency scores of 4.48 ± 0.5 and 4.25 ± 0.55 respectively. Given the multicentric nature of the study, a multilevel analysis was essential to adjust for variability between centers, providing more accurate estimates of the impact of smart fridges. Sensitivity analyses were also conducted to assess the robustness of the findings against different methodological assumptions, such as various inclusion/exclusion criteria and data handling methods for missing data.
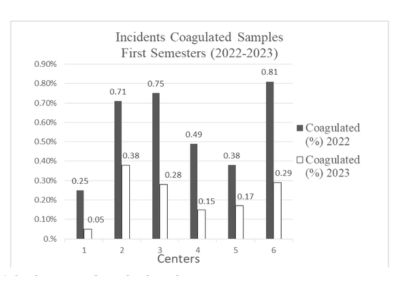
Figure 1. Incidence rates of coagulated samples.
This figure represents the incidences of coagulated samples relative to the total number of samples received. Centers using conventional refrigerators experienced reductions in the incidence of coagulated samples by 80.0%, 46.48%, and 34.67% in Centers 1, 2, and 3 respectively. In contrast, Center 4, which was equipped with smart technology, achieved a significant reduction from 0.49% to 0.15%, translating to a 69.39% decrease. To quantify these effects more precisely, logistic regression models were applied, adjusting for multiple covariates such as technology usage, center size, and staff training hours. The analysis revealed that the use of smart refrigerators is significantly associated with a reduction in the need to repeat tests (p < 0.001), as shown in Table 3.
The logistic regression analysis based on the adjusted dummy data shows that both the use of smart refrigerators and staff training hours have a significant impact on reducing the need to repeat blood tests. Larger centers tend to have a higher likelihood of pre-analytical errors, suggesting the need for specific strategies to address logistical and operational challenges in these settings. Implementing advanced technologies and investing in staff training are presented as effective measures to improve accuracy and efficiency in the pre-analytical phase of blood tests.
| Variable | Coefficient | OR (95% CI) | p-value |
| Intercept | 0.5 (0.1, 0.9) | 1.65 (1.10, 2.46) | 0,02* |
| Center Size | 0.01 (0.005, 0.015) | 1.01 (1.005, 1.015) | 0,04* |
| Training Hours | -0.02 (-0.03, -0.01) | 0.98 (0.97, 0.99) | 0,01* |
| Use of Smart Fridges | -0.5 (-0.75, -0.25) | 0.61 (0.47, 0.78) | <0.0001* |
| * = significant test | |||
Table 3. Logistic regression analysis on the need to repeat tests
The model’s constant has a coefficient of 0.50, indicating the baseline probability of repeating tests when all other variables are zero. The 95% confidence interval for this coefficient ranges from 0.10 to 0.90, with a p-value of 0.02. These results suggest that the constant is significantly different from zero, implying that there is an inherent probability of test repetition even in the absence of other factors. Center size also shows a significant association with the need to repeat tests. The coefficient for this variable is 0.01, with a 95% confidence interval ranging from 0.005 to 0.015 and a p-value of 0.04. A positive coefficient indicates that a larger center size is associated with a higher probability of needing to repeat tests. This may be due to the additional logistical challenges and greater operational complexity faced by larger centers. On the other hand, staff training hours are inversely related to the need to repeat tests. The coefficient for this variable is -0.02, with a 95% confidence interval ranging from -0.03 to -0.01 and a p-value of 0.01. This result indicates that more training hours are associated with a lower probability of needing to repeat tests, underscoring the importance of continuous staff training to improve accuracy and efficiency in the pre-analytical phase. The use of smart refrigerators is significantly associated with a reduction in the need to repeat tests, with a coefficient of -0.50. The 95% confidence interval for this variable ranges from -0.75 to -0.25, and the p-value is 0.0001. This negative coefficient suggests that the implementation of advanced technologies such as smart refrigerators improves pre-analytical efficiency and reduces errors, validating the effectiveness of this technology.
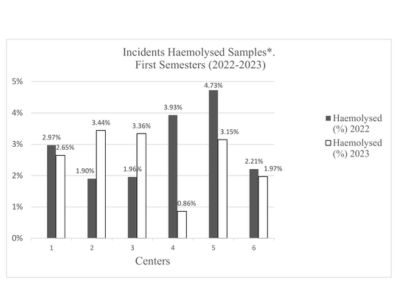
Figure 2. Incidence rates of hemolyzed samples.
Figure 2 represents the incidences of hemolyzed samples in relation to the total number of samples received. Centers 1, 5, and 6 experienced reductions of 10.77%, 33.40%, and 10.86%, respectively. Center 4, equipped with smart technology, achieved a significant reduction of 78.12%, (10.77% vs 78.12%, Z=14.20, p<0.001).
Figure 3 shows the incidences of hemolyzed samples in relation to the total number of samples received.
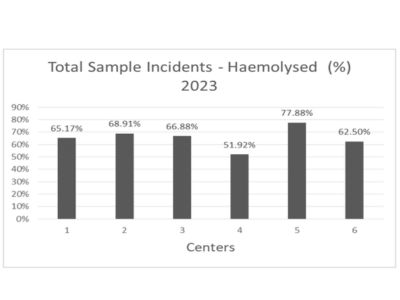
Figure 3. Incidence rates of hemolyzed samples over the total incidences.
Further analysis of the incidence relative to all samples from the first half of 2023, as shown in Figures 2 and 3, continues to demonstrate Center 4’s superior performance with lower rates of both coagulated (2.21%) and hemolyzed (51.92%) samples compared to other centers. These results underscore the effectiveness of smart refrigerators in reducing pre-analytical errors and improving the overall operational efficiency of diagnostic processes in health centers.
Healthcare Staff Satisfaction – Regarding the secondary objective focused on healthcare staff satisfaction with smart refrigerators, the data reveal a notably favorable perception. Although only 79.41% of the surveyed healthcare workers had direct experience with the smart refrigerators, the majority of these professionals reported very positive experiences (63.85%), with an additional 30.77% rating it positively. Significantly, no participants reported a negative perception, with the remaining percentage abstaining from making an assessment.
Refrigerator Quality Assessment – The overall quality of the refrigerators was highly rated, with an average score of 8.67 out of 10. These results highlight the acceptance and satisfaction with the technological innovations applied to the transportation of biological samples, promising not only to reduce pre-analytical errors but also to improve the overall operational efficiency of diagnostic
processes in health centers.
DISCUSSION
This study explores the incidence of pre-analytical errors during the transportation phase of biological samples, focusing on the use of smart refrigerators. Compared to centers without this technology, Center 4 demonstrated significant reductions in the rates of coagulated (69.39%) and hemolyzed samples (51.92%). These findings align with previous research emphasizing the need to optimize the pre-analytical phase to ensure diagnostic quality and patient safety [27]. The efficacy of smart refrigerators in mitigating pre-analytical errors is also supported by other studies highlighting the importance of proper sample management in mass spectrometry-based metabolomics studies [28]. The significant reduction in hemolyzed samples underscores the value of environmental control technologies in maintaining sample integrity during transportation. This is consistent with the findings of Lippi et al. [5], who indicate that proper management in the pre-analytical phase is crucial to minimize errors that affect laboratory results. Additionally, Alcantara et al. [30] discuss how pre-analytical errors, such as hemolysis, significantly contribute to variability in laboratory results, impacting diagnostic accuracy and reliability, thus reinforcing the need for technological interventions. In contrast, studies by Cornes [12] and Lee [11] address the improvement of pre-analytical errors in laboratories without advanced technologies.
However, it is important to acknowledge the primary investigational weakness of this study: the reliance on a single surrogate endpoint, the reduction in hemolyzed samples, to assess the effectiveness of the new system for sample transportation. While this endpoint showed significant improvement, the other chosen surrogate endpoint, the reduction in coagulated samples, did not demonstrate similar statistical significance, limiting the comprehensiveness of the study’s findings. Further, despite efforts to gather more comprehensive data, conclusions regarding the impact on other pre-analytical errors remain preliminary. The value of the Z statistic used in this study confirms the significance of reductions in hemolyzed samples and, as suggested by West et al. [31], indicates the need for more refined methodologies to capture and analyze pre-analytical data, which could enhance assessments of the impact of smart refrigerators. The internal consistency of the questionnaires administered to healthcare professionals, measured by Cronbach’s alpha, demonstrated high reliability, with values of 0.87 and 0.91 for the initial and final questionnaires, respectively. These results suggest that both questionnaires were reliable and consistent instruments, validating the study’s methodological robustness and reinforcing confidence in the findings obtained.
Regarding healthcare professionals’ perceptions of the challenges and critical areas in the pre-analytical phase of blood sample analysis, the majority of respondents (54.64%) indicated that it is “sometimes” necessary to repeat blood tests due to errors, highlighting the frequency of pre-analytical problems in their daily routine. The main sources of errors identified were sample transportation (24.74%), sample collection (23.71%), and storage conditions (19.59%). These areas were highlighted as the most critical points where errors occur, emphasizing the need for specific interventions [32]. Measures suggested to prevent errors in blood analysis included consideration of available material resources (29.90%) and staff training (23.71%), highlighting the importance of these factors in improving the accuracy and reliability of analyses [10]. When evaluating the current system of sample storage and transportation, the majority of respondents rated it as “Good” (55.67%), while 16.49% rated it as “Fair” and 8.25% as “Excellent”. Additionally, a notable proportion (16.49%) was unsure or did not respond. Most respondents (84.54%) believed that greater control of variables influencing sample transportation and storage would positively impact error reduction, underscoring the importance of strict controls in these areas to improve the accuracy and reliability of blood analyses [12]. The perceived need for test repetition significantly impacts both patients’ lives and the health center’s operations, with average scores of 7.049 and 7.516, respectively. Evaluations also highlight handling and transportation as critical pre-analytical sub-phases requiring immediate attention, with urgency scores of 4.48 and 4.25, respectively.
Given the multicenter nature of the study, a multilevel analysis was conducted to adjust for variability between centers, providing more accurate estimates of the impact of smart refrigerators. Sensitivity analyses were also performed to assess the robustness of findings against different methodological assumptions, such as various inclusion/exclusion criteria and methods of handling missing data [31].
The incidence rates of coagulated samples significantly decreased in centers using conventional refrigerators, with reductions of 80.00%, 46.48%, and 34.67% in Centers 1, 2, and 3, respectively. In contrast, Center 4, equipped with smart technology, achieved a significant reduction from 0.49% to 0.15%, representing a 69.39% decrease. To quantify these effects more precisely, logistic regression models were applied, adjusting for multiple covariates such as technology use, center size, and staff training hours. The analysis revealed that the use of smart refrigerators is significantly associated with a reduction in the need for repeat tests (p < 0.001). The logistic regression model based on adjusted data shows that both the use of smart refrigerators and staff training hours significantly impact the reduction of repeat blood test needs. Larger centers tend to have a higher likelihood of pre-analytical errors, suggesting the need for specific strategies to address logistical and operational challenges in these environments. Implementing advanced technologies and investing in staff training emerge as effective measures to improve accuracy and efficiency in the pre-analytical phase of blood analysis [10].
Regarding healthcare staff satisfaction with smart refrigerators, the data reveals a notably favorable perception. Although only 79.41% of surveyed healthcare workers had direct experience with smart refrigerators, most of these professionals reported very positive experiences (63.85%), with an additional 30.77% rating them positively. Significantly, no participant reported a negative perception, with the remaining percentage abstaining from evaluation. The overall quality of the refrigerators was highly valued, with an average score of 8.67 out of 10. These results highlight the acceptance and satisfaction with technological innovations applied to the transportation of biological samples, promising not only to reduce pre-analytical errors but also to improve the overall operational efficiency of diagnostic processes in health centers [27].
CONCLUSION
This study confirms that the implementation of smart refrigerators significantly reduces the incidence of pre-analytical errors, particularly hemolyzed samples, during the transportation phase of biological samples. The results align with prior research emphasizing the importance of optimizing the pre-analytical phase to ensure diagnostic quality and patient safety [27]. Smart refrigerators proved effective in mitigating errors by maintaining environmental control, as evidenced by significant reductions in hemolyzed samples [28].
The internal consistency of the administered questionnaires, with high Cronbach’s alpha values, validates the reliability of the instruments used to gather data on pre-analytical issues and healthcare staff satisfaction. The consistent responses highlight the robustness of the study’s methodology and reinforce confidence in the findings obtained.
Healthcare professionals identified transportation, collection, and storage conditions as the primary sources of pre-analytical errors. These insights underscore the need for targeted interventions in these areas. Suggested measures to prevent errors included improving material resources and enhancing staff training, emphasizing the critical role these factors play in ensuring the accuracy and reliability of blood analyses [10].
The logistic regression analysis demonstrated that both the use of smart refrigerators and increased staff training significantly reduce the need for repeat tests, particularly in larger centers where logistical challenges are more pronounced [10]. The study also revealed a notably favorable perception among healthcare staff regarding the effectiveness of smart refrigerators, with high satisfaction scores further validating their utility [27].
LIMITATIONS
This study on the efficacy of smart refrigerators in mitigating preanalytical errors during the transport and storage of biological samples identifies several key limitations that must be considered: Monitoring Period: The study was conducted from January to July 2023, excluding the hotter months of August and September. The absence of these months may overlook how extreme temperatures could affect the performance of smart refrigerators in maintaining sample integrity. This limits the ability to generalize the results to more adverse climatic conditions, which could have a significant impact on preanalytical errors. Generalizability of Results: The research was limited to a small number of primary care centers within the Elche Health Department. While the results are encouraging, their applicability to other settings with different logistical infrastructures and climates may vary, suggesting caution in broader extrapolation of these findings.
Variability in Implementation and Use: There is inherent variability in how technology is adopted and used across different centers. Assumptions of uniform training and homogeneous management of technology might not hold universally, potentially affecting the replicability of the results. Variability in staff training and operational procedures may introduce differences in the effectiveness of smart refrigerators.
Subjective Data: Much of the qualitative data derives from self-reported questionnaires and interviews, which are susceptible to biases such as the social desirability effect. This could influence the accuracy of reported perceptions regarding preanalytical issues and satisfaction with smart refrigerators. These data should be interpreted cautiously and considered complementary to the quantitative findings.
Confounding Variables: Despite rigorous protocols to control confounding factors, there are uncontrolled variables, such as equipment maintenance or workflow variations, that could influence the outcomes. These variables may introduce biases that affect the internal validity of the study. It is crucial to acknowledge these limitations and consider their potential impact on the findings.
These limitations, while noteworthy, do not diminish the value of the findings but highlight areas for future research to refine and validate the benefits of the technology in diverse settings. Future studies should include longer and more varied monitoring periods, a broader diversity of study centers, and a more thorough evaluation of the implementation and use of technology practices to ensure the generalizability and robustness of the results.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
Authors’ contribution
Collective authorship responds to a joint contribution in all section.
Conflict of interest
The authors report no conflict of interest.
REFERENCES
- Cakirca G. The evaluation of error types and turnaround time of preanalytical phase in biochemistry and hematology laboratories. Iran J Pathol. 2018;13(2):173–8. doi: 10.30699/IJP.13.2.173.
- Fraga OR, Segarra XN, Ortega AG, Cantalejo FR, Rioja RG, Queral LA, et al. Preanalytical recommendations for the measurement of acid-base balance and blood gases. Rev Lab Clin. 2019;12(4):e66-e74. doi: 10.1016/J.LABCLI.2018.12.001.
- Giavarina D, Lippi G. Blood venous sample collection: Recommendations overview and a checklist to improve quality. Clin Biochem. 2017;50(10-11):568-573. doi: 10.1016/j.clinbiochem.2017.02.021.
- Rak-Pasikowska A, Goniwiecha D, Bil-Lula I. Analysis of preanalytical phase errors in a medical diagnostic laboratory. Diagnostyka Laboratoryjna. 2019. doi: 10.5604/01.3001.0013.7440.
- Carraro P, Zago T, Plebani M. Exploring the initial steps of the testing process: frequency and nature of pre-preanalytic errors. Clin Chem. 2012;58(3):638-42. doi: 10.1373/clinchem.2011.175711.
- Lippi G, Betsou F, Cadamuro J, Cornes M, Fleischhacker M, Fruekilde P, et al. Preanalytical challenges – time for solutions. Clin Chem Lab Med. 2019;57(7):974–81. doi: 10.1515/cclm-2018-1334.
- Gosselin RC, Marlar RA. Preanalytical variables in coagulation testing: setting the stage for accurate results. Semin Thromb Hemost. 2019;45(5):433-448. doi: 10.1055/s-0039-1692700.
- Stankovic AK, DiLauri E. Quality improvements in the preanalytical phase: focus on urine specimen workflow. Clin Lab Med. 2008;28(2):339–50, viii. doi: 10.1016/j.cll.2007.12.011.
- Tóth J, Oláh AV, Petercsák T, Kovács T, Kappelmayer J. Detection of haemolysis, a frequent preanalytical problem in the serum of newborns and adults. EJIFCC. 2020;31(1):6–14. PMID: 32256284 PMCID: PMC7109500.
- Akande T. Quality Management of the Pre-Analytical Phase of Total Laboratory Testing Process: Monitoring and Control. Curr J Appl Sci Technol. 2018. doi: 10.9734/CJAST/2018/43874.
- Lee NY. Reduction of pre-analytical errors in the clinical laboratory at the University Hospital of Korea through quality improvement activities. Clin Biochem. 2019;70:24–29. doi: 10.1016/j.clinbiochem.2019.05.016.
- Cornes M. The preanalytical phase–Past, present and future. Ann Clin Biochem. 2020;57(1):4-6. doi: 10.1177/0004563219867989.
- Rodríguez-Ravelo MA, Marcel EA. Preanalytical variables and their influence on clinical laboratory results. Rev Mex Patol Clin. 2007;54(4):159-167. Available from: [https://www.medigraphic.com/pdfs/patol/pt2007/pt074c.pdf](https://www.medigraphic.com/pdfs/patol/pt-2007/pt074c.pdf).
- Vacutainer. CPT™ Cell Preparation Tube with Sodium Heparin For the Separation of Mononuclear Cells from Whole Blood Sterile Interior 8 mL Draw Capacity [16 x 125mm tube Size] For In Vitro Diagnostic Use. 2011. Available from: [https://www.bdbiosciences.com/content/dam/bdb/marketingdocuments/PI_CPT_heparin_March_2016_VDP4010507_web_500010323.pdf](https://www.bdbiosciences.com/content/dam/bdb/marketingdocuments/PI_CPT_heparin_March_2016_VDP4010507_web_500010323.pdf).
- Lippi G, von Meyer A, Cadamuro J, Simundic AM. Blood sample quality. Diagnosis. 2019;6(1):25–31. doi: 10.1515/dx-2018-0018.
- Tian G, Wu Y, Jin X, Zeng Z, Gu X, Li T, et al. The incidence rate and influence factors of hemolysis, lipemia, icterus in fasting serum biochemistry specimens. PLoS One. 2022;17(1):e0262748. doi: 10.1371/journal.pone.0262748.
- Bostic G, Thompson R, Atanasoski S, Canlas C, Hong Y, Kolins M, et al. Quality Improvement in the Coagulation Laboratory: Reducing the Number of Insufficient Blood Draw Specimens for Coagulation Testing. Lab Med. 2015;46(4):347–35. doi: 10.1309
- Lippi G, Salvagno GL, Montagnana M, Guidi GC. Short-term venous stasis influences routine coagulation testing. Blood Coagul Fibrinolysis. 2005;16(6):453-458. doi: 10.1097/01.mbc.0000178828.59866.03.
- Geest-Daalderop J, Mulder A, Winter L, Hoekstra M, Besselaar A. Preanalytical variables and off-site blood collection: influences on the results of the prothrombin time/international normalized ratio test and implications for monitoring of oral anticoagulant therapy. Clin Chem. 2005;51(3):561-8. doi: 10.1373/clinchem.2004.043174.
- Gosselin RC. Review of coagulation preanalytical variables with update on the effect of direct oral anticoagulants. Int J Lab Hematol. 2021;43:109-116. doi: 10.1111/ijlh.13585.
- Plebani M. Quality in laboratory medicine: 50 years on. Clin Biochem. 2016;50(3):101-104. doi: 10.1016/j.clinbiochem.2016.10.007.
- Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med. 2006;44(6):750–759. doi: 10.1515/CCLM.2006.123.
- Senes M, Bercik Inal B, Benli Aksungar F, Cinaroglu I, Eker P, Sonmez C, Yucel D. TBS preanalytical phase working group survey study–preanalytical phase in coagulation laboratories. Turk J Biochem. 2021;46(1):13-21. doi: 10.1515/tjb-2019-0512.
- Patel S, Nanda R, Sahoo S, Mohapatra E. Congruity in Quality Indicators and Laboratory Performance. Indian J Clin Biochem. 2018;33(3):341-347. doi: 10.1007/s12291-017-0687-9.
- Wallin O, Söderberg J, Grankvist K, Jonsson P, Hultdin J. Preanalytical effects of pneumatic tube transport on routine haematology, coagulation parameters, platelet function and global coagulation. Clin Chem Lab Med. 2008;46(10):1443-9. doi: 10.1515/CCLM.2008.288.
- Kim SJ, Yoo S, Kim HO, Bae H, Park J, Seo KJ, Chang B. Smart Blood Bag Management System in a Hospital Environment. 2006. doi: 10.1007/11872153_44.
- Lippi G, Banfi G, Church S, Cornes M, De Carli G, Grankvist K, Kristensen GB, Ibarz M, Panteghini M, Plebani M, Nybo M, Smellie S, Zaninotto M, Simundic AM. Preanalytical quality improvement: In pursuit of harmony, on behalf of European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working group for Preanalytical Phase (WG-PRE). Clin Chem Lab Med. 2015;53(3):357–370. doi: 10.1515/cclm-2014-1051.
- Chaudhry AS, Inata Y, Nakagami-Yamaguchi E. Quality analysis of the clinical laboratory literature and its effectiveness on clinical quality improvement: a systematic review. J Clin Biochem Nutr. 2023;73(2):108–115. doi: 10.3164/jcbn.23-22.
- Ren J, Zhang A, Kong L, Wang X. Advances in mass spectrometry-based metabolomics for investigation of metabolites. RSC Adv. 2018;8:22335-22350. doi: 10.1039/c8ra01574k.
- Alcantara J, et al. Analysis of preanalytical errors in a clinical chemistry laboratory: a 2-year study. Medicine. 2022;101(1):e29853. doi: 10.1097/MD.0000000000029853.
- West J, et al. Preanalytical errors in medical laboratories: a review of the available methodologies of data collection and analysis. Ann Clin Biochem. 2017;54(1):14-19. doi: 10.1177/0004563216669384.
- Peck Palmer OM, Dasgupta A. Review of the Preanalytical Errors That Impact Therapeutic Drug Monitoring. Ther Drug Monit. 2021;43(6):595-608. doi: 10.1097/FTD.0000000000000901.
![]() This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
