Salvatore Coppolino1*, Veronica Crucitti1, Febronia Federico1,
Emanuele Leotta1, Nadia Caporlingua2
- Unità Operativa Semplice (U.O.S.) Farmacia, Presidio Ospedaliero Barone Ignazio Romeo, Messina (Italy).
- Unità Operativa Semplice (U.O.S.) Farmacia, Presidio Ospedaliero Mistretta, Messina (Italy).
* Corresponding author: Salvatore Coppolino, Dirigente Farmacista, Presidio Ospedaliero Barone Ignazio Romeo, Via Giuseppe Mazzini, 14 Patti (Me) sacoppolin@yahoo.it
Cite this article
ABSTRACT
Introduction: Antimicrobial resistance is a global problem caused by the inappropriate use of antibiotics. To combat this phenomenon, multidisciplinary antimicrobial stewardship programmes have been initiated, the primary objectives of which include improving both the level of appropriateness of prescription and clinicians’ awareness of the correct use of antibiotics.
Objective/Purpose: The objective of the work was to conduct an analysis of antibiotic consumption at hospital level to assess the appropriateness of antibiotic prescription in a number of Operational Units.
Method: From 01/01/2021 to 31/12/2022, data were extracted from the Pharmacy Operational Unit’s management software on the dispensing of antibiotics (injectable and oral use), the Defined Daily Doses, and the number of dosage units dispensed to the General Medicine, Intensive Care, General Surgery and Neurorehabilitation Operational Units of two hospitals.
Results: Total antibiotic consumption was 7,845 dosage units in 2021 and 10,182 in 2022. The comparison of the defined daily dose values (4,565,485 in 2021 and 5,079,671 in 2022) is indicative of the use of antibiotics with different dosages, with a percentage increase of 11.3%. A comparison was also made between the Defined Daily Doses/100 bed-days delivered in 2021 and the regional and national figures, yielding a significantly lower figure than these latter figures.
Conclusions: The loss of antibiotic efficacy threatens to throw healthcare systems into crisis, leading to in an increase in morbidity and mortality from infections. The implementation of antimicrobial stewardship programmes remains, at present, the best tool to harness in order to curb the phenomenon of antimicrobial resistance. There is therefore a need for increasingly specialised professionals in the field of infectious diseases. Nurses and pharmacists play a crucial role in antimicrobial stewardship programmes, as they collaborate not only in the implementation of antimicrobial guidelines, but also in the review of individual patient regimens in order to optimise treatment and in the training of healthcare personnel on the appropriate use of antimicrobials.
Keywords: antimicrobial resistance, antimicrobial stewardship, antibiotics, appropriateness of prescription
INTRODUCTION
Antimicrobial resistance is a growing global public health problem that could lead, unless action is taken, to 10 million deaths a year by 2050 [1,2].
It is a complex phenomenon with a multifactorial genesis: the increased use of antibiotics (including inappropriate use), the spread of hospital infections with antibiotic-resistant micro-organisms (and the limited control of these infections), and the increase in international travel with the consequent increased spread of strains. Many pathogens are also simultaneously resistant to several classes of antibiotics (multidrug resistance) [3].
The resistance of bacteria to antibiotics can be divided into two types: natural (or innate) resistance and acquired resistance.
Acquired resistance is the result of clonal selection due to the selective pressure exerted by the drug and can be broken down into chromosomal resistance, which accounts for about 10-15% of all resistance, and extra-chromosomal resistance, which accounts for about 90% of all resistance and is mediated by gene sequences in plasmids or transposons (mobile genetic elements) [4,5].
The mechanisms whereby microorganisms become resistant to antibiotics include the production of antibiotic-inactivating enzymes (the production of ꞵ-lactamases, for example, is one of the most widespread resistance mechanisms. The enzyme hydrolyses the ꞵ-lactam ring, a pharmacophore, resulting in the loss of activity of the molecule); altered envelope permeability; altered targeting (altering the DNA gyrase by substituting a single amino acid makes the enzyme resistant to quinolone antibiotics); active transport systems (protein-coding genes that act as efflux pumps for antibiotics) and alternative metabolic pathways.
The Italian periodic national surveillance report (AR-ISS), published in 2021 by the Istituto Superiore di Sanità, highlighted the main pathogens (Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter species) responsible for the phenomenon of antimicrobial resistance, the use of which should be closely monitored.
In 2021, 33.1% of Klebsiella pneumoniae isolates and 8.8% of Escherichia coli isolates were multi-resistant to third-generation cephalosporins, aminoglycosides and fluoroquinolones; for Pseudomonas aeruginosa, the percentage of resistance to three or more antibiotics (piperacillin/tazobactam, ceftazidime, carbapenems, aminoglycosides and fluoroquinolones) was 11.4%; a high percentage of multi-resistance (fluoroquinolones, aminoglycosides and carbapenems) (85.4%) was observed for Acinetobacter species. The national figures on carbapenem-resistant Enterobacteriaceae (CRE) infections reported as many as 2,192 cases in 2021, confirming the widespread occurrence of carbapenem-resistant Enterobacteriaceae bacteraemia in Italy, especially in hospitalised patients [6].
Inappropriate use of antibiotics is associated with unfavourable outcomes, such as death, treatment failure and adverse reactions, resulting in an increased burden of care on the healthcare system, duration of antibiotic therapy and duration of hospitalisation [7,8]. It
also promotes the selection of resistant strains and the spread of antimicrobial resistance [9,10].
In Italy, the high levels of antibiotic resistance and antibiotic consumption require urgent prevention and monitoring action. Despite the downward trend, consumption continues to be higher than the European average, both in the human and veterinary sectors, with considerable interregional variability. Furthermore, in European maps of the distribution of resistant bacteria in Europe, Italy holds, together with Greece, the record for the spread of resistant germs. One of the main causes behind the increase in resistance in Italy and worldwide is the excessive use of antibiotics, especially after the emergency linked to the COVID-19 pandemic. To combat antimicrobial resistance, so-called “Antimicrobial Stewardship” (AS) [11] programmes have been initiated. These represent a series of interventions aimed at promoting and monitoring the correct choice of antibiotic, dosage and duration of treatment to preserve the future effectiveness of these molecules in real-life everyday clinical practice [12,13].
The Antimicrobial Stewardship Team (AST) is a multidisciplinary corporate body that must include an infectious disease specialist, a hospital pharmacist, a hygiene specialist, a nurse and a clinical microbiologist [13,14].
The primary objectives are to improve the level of appropriateness of prescription and clinical and microbiological indicators, to increase awareness among healthcare professionals on the correct use of antibiotics and the prevention of care-related infections, and to reduce costs due to short- and long-term clinical complications. Each member of the AST Team must cooperate with the corporate Hospital Infection Committee (HIC) for the activities within their competence. The resolution establishing the AST must define the responsibilities and methods of cooperation with the corporate HIC. It is of paramount importance that there is no ambiguity of roles between the AST and the HIC at corporate level, and that both maintain well-defined and closely interconnected areas of activity established by each individual health authority.
At local level, the Sicily Region, with Local Government Decree no. 703 of 04/08/2020, has drawn up a regional guideline document for the organisation of corporate AS programmes, setting out the lines of action to be followed by all companies. These include the implementation of the antimicrobial stewardship corporate function and the appointment of departmental representatives for each individual Operational Unit, represented by a doctor and a nurse, increased audits and feedback on the appropriateness of prescription, the establishment of lists of high-concern antibiotics, the implementation of a structured and informed system for infectious disease consultancy, the preparation of semi-annual reports, the drafting of corporate protocols for antibiotic therapy and prophylaxis and empirical therapy in hospitalised patients, company training and impact assessments [15].
With the CRE Regional Directive (DASOE/8/21932), in implementation of the circular of the Ministry of Health no. 1479 of 17/01/2020, the Sicily Region has initiated a surveillance programme for bacteraemias caused by carbapenem-resistant Enterobacteriaceae (CRE). The Directive provides for the collection of case reports on the regional territory, data analysis, monitoring, dissemination and evolution of infections, with periodic transmission of the data to the Italian National Health Institute (Istituto Superiore di Sanità) [16].
All the actions undertaken by the Sicily Region form part of the 2020-2025 Regional Prevention Plan, which represents the main planning, prevention and health promotion tool, as it places the citizen at the centre of the interventions, accompanying them throughout all phases of life, with the aim of achieving the highest level of health. The Regional Plan envisages, by 2025, the establishment of AST in all Regional Health Authorities [17].
Objective/Purpose
The objective of the work was to conduct an analysis of the consumption of antibiotics in certain Operational Units at the “Barone Ignazio Romeo” Hospital in Patti and the “San Salvatore” Hospital in Mistretta, part of the Provincial Health Authority of Messina, to assess the appropriateness of their use per Operational Unit.
MATERIALS AND METHODS
Sampling and eligibility
A retrospective observational analysis was conducted between 01/01/2021 and 31/12/2022.
The data were obtained from the consumption of antibiotics provided by the hospital pharmacies at the hospitals concerned in the five Operational Units considered.
Tools
For the implementation of this study, paper prescription forms for injectable antibiotics and the consumption of both antibiotics administered both intravenously (i.v.) and orally (p.o.), provided by the Pharmacy Operational Units of the two hospitals in Patti and Mistretta, were considered.
The injectable antibiotic prescription form used for administration bears the patient’s initials, the required active substance, the number of vials, the dosage, the duration of treatment, and whether it is targeted treatment or empirical treatment. The data on the dispensing of antibiotics by the two Pharmacy Operational Units, both for injection and oral use, to the General Medicine, Intensive Care, General Surgery and Neurorehabilitation Operational Units of the Patti and Mistretta Hospitals, facilities belonging to the Messina Provincial Health Authority (ASP), were extracted from the corporate management software. The number of beds per single Operational Unit and per year are shown in Table 1.

Table 1. Numbers of beds in different Operational Units at Patti and Mistretta hospitals
The data were collected by the researchers and processed in aggregate form for research purposes only. All the authors who took part in the observational study are hospital pharmacists, some with twenty years of service and a PhD in Pharmaceutical Sciences. All the authors have obtained the Specialisation in Hospital Pharmacy or in Pharmacology and Clinical Toxicology. To ensure confidentiality, each patient was assigned a number. For all the antibiotics dispensed, the Defined Daily Doses (DDDs) were considered, i.e. the average doses taken daily by an adult patient, with reference to the main therapeutic indication of the drug [18] and the number of dosage units dispensed to the individual Operational Units. Consumption was calculated as DDD/100 bed-days according to the scheme used by the Italian National Observatory on the Use of Medicines (OsMed) in the national report on the use of antibiotics in Italy for the year 2021[19].
Statistical Analyses
The data have been presented as numbers and percentages for categorical variables and in terms of the arithmetic mean in the case of continuous variables. For the management of our data, the calculation of DDDs, Delta (2022-2021) and Pareto diagrams were carried out using an Excel spreadsheet. In particular, the Pareto diagram was used to understand which factors could most influence our results.
RESULTS
Total antibiotic consumption was 7,845 dosage units in 2021 and 10,182 in 2022. The comparison of the DDD values (4,565,485 in 2021 and 5,079,671 in 2022) is indicative of the use of antibiotics at different dosage strengths, as an increase in DDD corresponds to an increase in the doses administered at different dosage strengths, allowing a comparison of consumption as it is a technical tool for measuring drug prescriptions. In 2022, there was a percentage increase of 11.3%. The analysis of DDDs was carried out on 100 bed-days provided per individual Operational Unit.
For Patti Hospital (Table 2), for the General Surgery department, 485,762 DDDs were provided in 2021 and 784.869 in 2022; for General Medicine, 1,817,933 DDDs in 2021 and 2,354,803 DDDs; for Intensive Care, 598,954 DDDs in 2021 and 736.244 DDDs in 2022; while for the Operational Unit of Mistretta Hospital (Table 3), 1,249,067 were provided for the General Medicine department in 2021 and 433.301 for 2022 and for the Neurorehabilitation department, 414.488 DDDs in 2021 and 770.457 DDDs in 2022.



Table 2. Comparison of antibiotic consumption in 2021 and 2022 at Patti Hospital.

Table 3. Comparison of antibiotic consumption in 2021 and 2022 at Mistretta Hospital.
For the year 2021, the consumption of DDD/100 bed-days in the Operational Units at the two hospitals examined was compared with the regional data and with the national data based on the findings of the 2021 National Report “The use of antibiotics in Italy” produced by OsMed [20]. The DDD/100 bed-days provided in 2021 was 70.6 at national level; in the Sicily Region it was 81.6, while in the Operational Units we examined it was 44.3.
The analysis of the data shows for Patti Hospital an increase in the consumption of amoxicillin/clavulanic acid, both p.o. and i.v. (Δ%=0.47), piperacillin/tazobactam (Δ%=1.23) and levofloxacin (Δ%=0.5) in General Surgery. An increase in the use of third-generation cephalosporins (Δ%=1.68), ciprofloxacin, both p.o. and i.v. (Δ%=1.21%), gentamicin (Δ%=0.73), linezolid and (Δ%=0.2) piperacillin/tazobactam (Δ%=1.54) was observed in General Medicine. Finally, an increase in the use of third-generation cephalosporins (Δ%=0.81), ciprofloxacin (Δ%=0.2), gentamicin (Δ%=0.06) and piperacillin/tazobactam (Δ%=1.35) was also observed in Intensive Care.
For Mistretta Hospital, there was no significant difference in Δ% between 2021 and 2022, only a clear reduction in the consumption of ceftriaxone (Δ% = -6) in General Medicine and an increase in amoxicillin/clavulanic acid, both p.o. and i.v. (Δ%=0.98%), and an increase in ertapenem (Δ%=0.61%) in Neurorehabilitation. From the prescription forms viewed, it was found that empirical prescriptions correspond to 92.8%, while those based on antibiogram evaluation only account for 7.2% of total prescriptions. Parenteral Amoxicillin/Clavulanic acid is used for otitis, sinusitis and COPD (chronic obstructive pulmonary disease), Cefazoline for respiratory tract infections and peritonitis, ceftazidime for gram-negative bacteria and Pseudomonas infections and for surgical interventions; ciprofloxacin for COPD, respiratory and urinary tract infections; gentamicin as a broad-spectrum antibiotic; meropenem for staphylococcal and streptococcal infections; piperacillin/tazobactam for pneumonia; teicoplanin for resistant Staphylococcus aureus infections. Further analysis was carried out on antibiotics for injectable use that required the use of prescription forms to monitor their appropriate use. The following antibiotics were considered: ciprofloxacin, colistin, fosfomycin, imipenem/cilastatin, levofloxacin, linezolid, meropenem, teicoplanin, tigecycline and vancomycin.
A comparison between 2021 and 2022 was performed using the Pareto diagram (Figure 1 and 2).

Figure 1. Pareto diagram of injectable antibiotics under monitoring for the years 2021 and 2022.
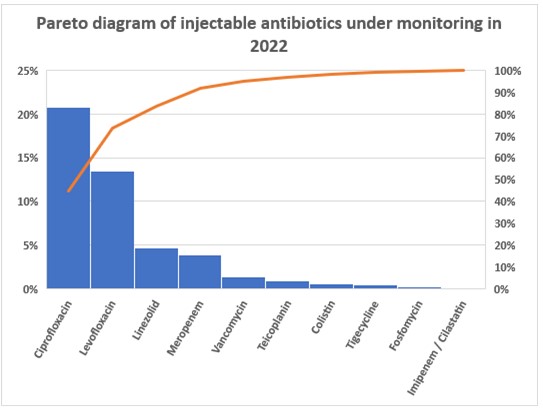
Figure 2. Pareto diagram of injectable antibiotics under monitoring for the years 2021 and 2022.
The Pareto diagrams showed that in 2022, there was a reduction in prescriptions for ciprofloxacin, levofloxacin and tigecycline. Increases were found for linezolid and meropenem. For the other antibiotics, prescriptions have remained constant or have undergone slight increases.
DISCUSSION
The analysis of paper prescription forms shows a consumption based more on empirical treatment (92.8%) than on antibiogram evaluations (7.2%), a situation that has prompted further corrective action by the AST and HIC, such as compulsory antibiograms for the antibiotics vancomycin, tigecycline, teicoplanin, meropenem, linezolid and the combination imipenem/cilastine.
Furthermore, for high-cost injectable antibiotics, such as fosfomycin or the meropenem/vaborbactam combination, a specific prescription form has been drawn up, in which certain requirements must be fulfilled by the patient to be eligible for that treatment, so that these antibiotics are reserved for cases where there are no valid treatment alternatives. Analysis of the Pareto diagrams showed that the use of the prescription form proved to be a valuable tool for improving appropriateness of prescription.
Although COVID-19 put the appropriate use of antibiotics at risk, especially in the early stages of the pandemic – for instance the improper use of azithromycin – hospital pharmacists and nursing coordinators worked hard to draw up and implement internal protocols on the use of antibiotics in Operational Units to ensure their rational use.
Hospital pharmacists and nursing coordinators play a crucial role in antimicrobial stewardship (AS) programmes, as they are involved in the development and management of antimicrobial guidelines, the review of individual patient regimens to optimise treatment, and the training of healthcare personnel on the appropriate use of antimicrobials.
The results from the cohort examined show that to ensure appropriate use and targeted treatment, thus avoiding the phenomenon of antibiotic resistance, it is necessary to isolate the bacterium responsible for the infection and carry out an antibiogram before administering certain antibiotics to hospitalised patients. Specifically, this procedure concerns vancomycin, tigecycline, teicoplanin, meropenem, linezolid, levofloxacin, the imipenem/cilastatin combination, fosfomycin, colistimethate and ciprofloxacin.
The AS strategy, which has been followed in our hospitals to date, involves a careful and thorough patient assessment, the choice of the most suitable antimicrobial to be prescribed, its administration and the monitoring of the patient once treatment has begun. This last phase includes the possibility of reducing the duration of antimicrobial treatment, converting the route of administration from intravenous to oral, modifying the dose based on any clinical conditions that might affect the patient’s pharmacokinetics, such as excretory system deficiencies, monitoring the use of the prescribed antibiotic, monitoring adherence to treatment, and informing the patient on the appropriate use of antimicrobials [21,22]. The hospital pharmacist plays a crucial role in the fight against antibiotic resistance by acting as a link between the clinician and the microbiology laboratory. This surveillance activity results in a positive effect on how antibiotics are prescribed by healthcare professionals, leading to a reduction in hospital infections.
The use of antibiotics must also be monitored in real-life everyday clinical practice. The nursing coordinator collects and reports to the hospital pharmacist any adverse reactions to antibiotics to confirm or otherwise the initial risk/benefit ratio with which the drug was marketed.
CONCLUSIONS
The effects of resistance, i.e., the inability of antibiotics, administered at therapeutic doses, to reduce survival or inhibit the replication of pathogenic bacteria, can be observed worldwide. Recently, the phenomenon has been further aggravated by their often inappropriate use.
The implementation of AS programmes in all health authorities remains, at present, the best tool to harness in order to curb the phenomenon of antimicrobial resistance.
The discovery of new molecules with antimicrobial activity capable of treating infections by multiresistant microorganisms is not an immediate tool; years of preclinical and clinical studies are required for a new molecule to be marketed. The phenomenon of antimicrobial resistance is urgent and requires an immediate solution to combat it. Increasingly more specialised figures are needed in the field of infectious diseases than just clinicians, and therefore hospital pharmacists and nursing staff must also be adequately trained to be able to give their best possible support in the battle against antimicrobial resistance.
LIMITATIONS
The work conducted is based on prescription forms and consumption data of antibiotics used by injection or orally. The work considers a limited geographical area. Lastly, a further limitation of the study is the lack of inferential analysis of our data.
Ethical considerations
No formal approval by the Local Ethics Committee was necessary for this type of study, since it is a publication concerning consumption data and aggregated data.
No economic incentives were provided for this analysis. Authorisation for the use of prescription forms was issued by the Hospital’s Medical Director, the consumption data derive from reports certified by the Corporate Management Control. The participants’ anonymity was ensured. The study was conducted in accordance with the ethical considerations of the Declaration of Helsinki.
Funding statement
This research did not receive any specific contributions from public, commercial or non-profit funding bodies.
Conflicts of interest
The authors do not report any conflicts of interest.
Contributions of the authors
All authors contributed equally to the production of this study.
REFERENCES
- Akpan, M.R., Ahmad, R., Shebl, N.A., et al. A review of quality measures for assessing the impact of antimicro- bial stewardship programs in hospitals. Antibiotics (Basel). 2016;5(1):5.
- O’Neill, J. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance. 2016. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf. Last accessed on July 23, 2023.
- Garau, J., Nicolau, D.P., Wullt, B., et al. Antibiotic stewardship challenges in the management of community-acquired infections for prevention of escalating antibiotic resistance. J Glob Antimicrob Resist. 2014;2(4):245–53.
- Antimicrobial resistance (AMR) Disponibile su: http://www.ecdc.europa.eu/en/activities/surveillance/EARS-Net/. Last accessed on August 19, 2023
- World Health Organization. Antimicrobial resistance Global Report on surveillance 2014. http://www.who.int. Last accessed on August 08, 2023.
- Aspetti epidemiologici in Italia Sorveglianza nazionale dell’Antibiotico-Resistenza (AR-ISS) https://www.epicentro.iss.it/antibiotico-resistenza/epidemiologia-italia. Last accessed on August 19, 2023.
- Spoorenberg, V., Hulscher, M.E, Akkermans, R.P., Geer- lings SE. Appropriate antibiotic use for patients with urinary tract infections reduces length of hospital stay. Clin Infect Dis. 2014;58(2):164–9.
- Ortega, M., Marco, F., Soriano, A., Almela M., Martinez J.A., Munoz A. et al. Epidemiology and prognostic determinants of bacteraemic catheter-acquired urinary tract infection in a single institution from 1991 to 2010. J Infect. 2013;67(4):282–7.
- Dalfino, L., Bruno, F., Colizza, S., Concia E., Novelli A., Rebecchi F.S. et al. Cost of care and antibiotic prescribing attitudes for community acquired complicated intra-abdominal infections in Italy: a retrospective study. World J Emerg Surg. 2014; 9:39.
- Magill, S.S., O’Leary, E., Janelle, S.J., Thompson D.L., Dumyati G., Nadle J. et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N Engl J Med. 2018; 379(18):1732-1744
- Karki, T., Plachouras, D., Cassini, A., Suetens C. Burden of healthcare-associated infections in European acute care hospitals. Wiener Medizinische Wochenschrift. 2019; 169: 3–5.
- Goff, D.A., Rybak, M.J. Global antimicrobial stewardship: challenges and successes from frontlinestewards. Infect Dis Ther. 2015;4(Suppl 1):1–3.
- Society for Healthcare Epidemiology of America. Infectious dis- eases society of America, pediatric infectious diseases society. policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322–7.
- Dellit, T.H., Owens, R.C., McGowan, J.E., Gerding D.N., Weinstein R.A., Burke J.P. et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis.2007;44(2):159–77.
- European Centre for Disease Prevention and Control. Proposals for EU guidelines on the prudent use of antimicrobials in humans, 2017. https://www.ecdc.europa.eu/en/publications-data/proposals-eu-guidelines-prudent-use-antimicrobials-humans. Last accessed on July 23, 2023.
- Decreto Assessoriale n. 703 del 04/08/2020 della Regione Sicilia http://pti.regione.sicilia.it/portal/page/portal/PIR_PORTALE/PIR_LaStrutturaRegionale/PIR_AssessoratoSalute/PIR_Infoedocumenti/PIR_DecretiAssessratoSalute/PIR_DecretiAssessoriali/PIR_DecretiAssessorialianno2020/DA%20N.%20703.pdf. Last accessed on July 23, 2023.
- Direttiva Regionale CRE (DASOE/8/21932) https://www.qualitasiciliassr.it/sites/doc/cre/direttiva_cre_dasoe21392_29042021.pdf. Last accessed on July 23, 2023.
- Piano Regionale della prevenzione 2020-2025. http://www.quadernidellasalute.it/portale/prevenzione/DELIBERE_PRP_2020-2025/Sicilia/Allegato_1-PRP_23_12_2020-2025_integrato.pdf. Last accessed on July 23, 2023.
- Gli strumenti: il metodo di classificazione secondo il sistema ATC/DDD Bollettino d’informazione sui farmaci. Anno IX N. 6. http://www.agenziafarmaco.gov.it/wscs_render_attachment_by_id/111.61850.1150390484813676c.pdf?id=111.61855.1150390485109. Last accessed on August 08, 2023.
- L’uso degli antibiotici in Italia Rapporto Nazionale Anno 2021. https://www.aifa.gov.it/documents/20142/1853258/Rapporto_Antibiotici_2021.pdf Last accessed on August 08, 2023.
- Gilchrist, M., Wade, P., Ashiru-Oredope, D., Howard P., Sneddon J, Whitney L. et al. antimicrobial stewardship from policy to ptice: experiences from UK antimicrobial pharmacists. Infect Dis Ther. 2015;4(Suppl 1):51–64.
- Barlam, T.F., Cosgrove, S.E., Abbo, L.M., MacDougall C., Schuet A.N., SeptimusEJ., et al. Implementing an antibiotic stewardship pro- gram: guidelines by the infectious diseases Society of America and the society for healthcare epidemiology of America. Clin Infect Dis. 2016;62(10): e51–77.
![]() This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

